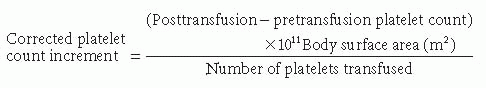Platelet Concentrates and Pheresis Platelets: Platelet transfusions are available as platelet concentrates or as apheresis units,
1 the former prepared from whole blood by centrifugation and the latter collected by pheresis devices. Platelet concentrates are separated from whole blood by first preparing platelet-rich plasma, from which the platelets are centrifuged. The contents of the platelet concentrates are highly variable, but 50 mL of platelet concentrate has at least 5.5 × 10
10 platelets. A buffy coat method uses centrifugation to prepare a buffy coat, from which platelets are separated by an additional centrifugation. The white cell contamination is approximately 10
8 per bag using the platelet-rich plasma method of concentration, but considerably lower (10
6 per bag) for platelets prepared by the buffy coat method. Lower white blood cell (WBC) content in buffy coat platelet preparations may be advantageous in reducing alloimmunization and febrile transfusion reactions.
1,2,3 Platelet concentrates obtained from platelet-rich plasma method versus the buffy coat method do not show any functional difference
in vitro, and comparative clinical trials have not been done.
The platelet storage lesion is characterized by (a) irreversible change in platelet shape from discoid to spherical, (b) generation of lactic acid from glycolysis, with an associated decrease in pH, (c) release of cytoplasmic and granule content causing a decrease in
in vitro measures of platelet function and ADPinduced shape change, and (d) reduction in
in vivo recovery and survival. Many of the biochemical changes are related not just to the active metabolism of platelets but to preparation technique and storage conditions. Consequently improvements in bag composition and platelet additive solutions that maintain platelet metabolism during storage are under evaluation.
2,4,5
Clinical Applications of Platelet Transfusions
Thrombocytopenia: Platelet transfusions in thrombocytopenic patients enhance endothelial support functions by plugging fenestrations between endothelial cells.
Table 78.1 outlines platelet transfusion guidelines for clinical situations, including issues of long-term support.
6,7,8 There has been considerable interest in defining the lowest safe platelet concentration at which bleeding is unlikely, so that fewer transfusions are consumed. Investigators have now shown that a prophylactic platelet transfusion threshold of 10,000/µL in adult patients with acute leukemia is safe,
9,10,11 thereby decreasing platelet utilization, with only a small adverse effect on bleeding and with no effect on mortality. Thus, prophylactic transfusions should be given to patients with amegakaryocytic thrombocytopenia if the count falls below 10,000/µL, and at values between 5 and 10,000/µL, transfusion can be held if the patient is stable and if no other conditions make spontaneous bleeding likely. These conditions include blast crisis, anticoagulation with heparin for disseminated intravascular coagulation (DIC), drugs that affect platelet function, uremia, and recent invasive procedures, including lumbar punctures or placement of central venous catheters. A study in patients after autologous peripheral blood stem cell transplantation demonstrated the safety and feasibility of therapeutic platelet transfusions, with one-third of transplants performed without platelet transfusions, reducing platelet transfusion by 50% compared with historical controls when the practice of prophylactic transfusions was in place.
11
Platelet Dosing: While there have been a few small studies addressing platelet dosing,
12,13,14 a well-designed dose-ranging study was recently completed.
15 In this large study by Slichter et al., 1,351 adult and pediatric hematology-oncology patients were randomized to receive low (1 × 10
11), medium (2.2 × 10
11), and high dose (4.4 × 10
11) platelets per m
2 body surface area. Among the 1,272 evaluable patients, WHO grade 2 or higher bleeding was not significantly different between the three groups. This study suggests that fewer transfused platelets can
prevent clinically relevant bleeding. It should however be noted that the total number of platelets transfused ended up being higher in the low-dose platelet group who received a median of five transfusion events versus three in the other two groups. Thus while the dose may be decreased it is not apparent that the total number of platelets needed to sustain a patient through a period of aplasia will be decreased.
15
Platelet Transfusion in Specific Disorders
Consumptive Thrombocytopenias: Thrombocytopenia may develop on the basis of increased consumption, for example, autoimmune or drug-induced thrombocytopenia, DIC, and thrombotic thrombocytopenic purpura (TTP). Thrombocytopenia in these conditions can be quite severe, and profound bleeding is not uncommon, so platelet transfusions may be considered necessary in such patients. In the immune-mediated thrombocytopenias, the destruction of allogeneic platelets is as likely as are autologous platelets, and platelet transfusion is not the only therapeutic means to treat this form of thrombocytopenia.
16 There has been no documentation of increased morbidity from using platelets in DIC despite some concern that their use may increase disease severity by fueling the ongoing activation of coagulation. In TTP, the use of platelet transfusions has been said to provoke acute clinical deterioration
17 but there are also cases in which patients received platelet transfusions without difficulty, although they were already undergoing plasma exchange therapy.
18
Qualitative Platelet Disorders: The most common acquired qualitative platelet abnormalities are either drug-induced or caused by uremia, others occurring in patients with myeloproliferative disorders, dysgammaglobulinemias, or secondary to mechanical cardiac assist devices. No controlled trials have been conducted to determine what an appropriate platelet dose should be, but one recommendation is for at least 10 platelet equivalent units of platelets should be given for severe bleeding or for extensive surgery.
19 Platelet transfusions can improve the bleeding time in patients taking aspirin if the transfused platelets constitute 10% of the platelet population,
20 an observation of importance in cardiac patients who require emergency intervention. Whether one can extend this observation to other antiplatelet drugs such as clopidogrel is unclear. In those patients where clopidogrel cannot be stopped preoperatively within 72 hours of surgery, blood loss is higher and platelet transfusions are frequently given.
21
Inherited qualitative platelet disorders such as Glanzmann thrombasthenia and Bernard-Soulier syndrome can be manifest by severe bleeding, particularly following trauma. Platelet transfusions are effective in these disorders until the patient becomes alloimmunized to human leukocyte antigens (HLAs)
or to a platelet glycoprotein receptor such as the IIb/IIIa or Ia/IX complex, after which platelet transfusions are ineffective. In this situation, recombinant factor VIIa has been used successfully.
22Platelet Transfusion Refractoriness: Failure to achieve an expected increment to a platelet transfusion (refractoriness) may be caused by immune or nonimmune processes, most commonly due to HLA alloantibodies.
23,24 Before labeling a patient as being refractory to platelet transfusions, one should document low platelet recovery after using ABO identical platelets on two sequential transfusions.
25 The platelet count increment is calculated using the following formula:
This formula takes into account the dose of platelets given in total number of platelets and is corrected for the body surface area of the patient. Facilitating this calculation is the labeling of the platelet bag with the number of platelets in the bag by the facility producing the platelet transfusion. An acceptable corrected platelet count increment should be >7,500 platelets per m2/µL within 1 to 60 minutes after the transfusion, and >4,500 platelets per m2/µL if measured 18 to 24 hours later.
Immunologic Basis of Platelet Transfusion Refractoriness: Serial platelet transfusions result in decreasing platelet count increments. These transfusion failures result from the induction of alloantibodies to HLA-A and -B locus antigens.
6 Antibodies to human platelet alloantigens such as HPA-1a (PLA1) are only rare causes of platelet transfusion refractoriness. ABO compatible transfusions are associated with about a 20% greater increment than those that are not compatible. Some patients with blood group O who have high isoagglutinin titers to group A or group B antigens present on platelets may also fail to achieve adequate platelet increments.
25
HLA antibodies are measured using lymphocytotoxicity or enzyme-linked immunosorbent assay (ELISA) testing. Some patients may have decreases in or a loss of HLA antibody, either permanently or transiently, yet still be successfully transfused with platelet concentrates. HLA antibodies may be transient, and after their disappearance, patients are not refractory to platelets, even though they are not HLA matched. Some patients may have antibodies to HLA class I and yet not have platelet transfusion failures, because these HLA antigens may not be well expressed on platelets.
26,27,28There are two broad types of HLA antibodies produced in response to platelet transfusions. The first type recognizes epitopes unique to a particular HLA allele, referred to as antibodies to private specificities and including A2 or B12. The second type of HLA antibodies recognize more than one gene product. These antibodies recognize structural similarities between gene products (cross-reactive epitopes) or identical epitopes present on different gene products of different alleles, and are referred to as antibodies to public epitopes. Traditionally, HLA serology has placed the greatest emphasis upon classifying the private antigens, although more attention has recently been given to the clinical importance of public HLA specificities, the best known examples of which are Bw4 and Bw6. These antigens are encoded by a diallelic system and are associated with two different groups of HLA-B class I antigens. Other public antigens carried by HLA-B class I antigens have been divided into four cross-reactive groups, B5, B7, B8, and B12. The observation that the specificity of HLA antibodies in multiply transfused individuals is generally against private epitopes suggests that matching for these public antigens is not necessary.
29,30,31Table 78.2 outlines alternative approaches to selecting platelets for an alloimmunized patient. In patients with an unusual HLA type, such as in some ethnic groups, HLA matching may be difficult. Relying only on HLA matching may have shortcomings and in some cases it is overly restrictive, in that some HLA-B loci antigens are not located on platelets. Therefore, in addition to antigen matching there has been interest in adopting an approach to red cell compatibility. In this paradigm, one first selects a red cell phenotype that matches the patient’s phenotype, and then performs a major crossmatch. This same approach can be used for platelets by also performing a platelet crossmatch. Unlike red cell pretransfusion testing, there are no accepted standards for selecting platelet transfusions in patients with alloantibodies to platelets.
23,24
Nonimmune Refractoriness: Platelet transfusions may not result in an increment if the stored platelets are defective. Splenomegaly is a major cause of platelet transfusion failure. Normally, approximately 30% of a patient’s platelet mass is contained within the spleen, but with increases in splenic size, up to 90% of circulating platelets can be sequestered. Characteristically, splenic sequestration is associated with a reduced 1-hour platelet recovery but a normal survival. Fever and infection also cause decreased platelet survival. One study noted that platelet transfusion requirements were increased by 50% in febrile patients, even more so in patients with major infections and DIC. Medications may cause platelet refractoriness, including amphotericin, vancomycin, antithymocyte globulin, granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and interferons.
32,33,34 Gamma radiation of platelet transfusions either prior to transfusion or prior to storage period does not affect platelet transfusion survival.
Management of Platelet Transfusion Refractoriness and Bleeding: When no compatible platelets can be found for an alloimmunized patient who is bleeding, or may be undergoing invasive procedures, therapeutic modalities have included splenectomy, corticosteroids, plasmapheresis, administration of intravenous gamma globulin, and repeated platelet transfusions. Except for intravenous immunoglobulin, there is little evidence that any of these work.
23,24,35 Kickler et al.
35 performed a randomized, placebo-controlled clinical trial, investigating the use of intravenous immunoglobulin (400 mg/kg for 5 days) in patients with alloimmunized thrombocytopenia. An incompatible platelet transfusion from the same donor was used before and after the patient received study drug or placebo. Although platelet recovery at 1 to 6 hours was satisfactory in five patients after intravenous immunoglobulin treatment, 24-hour survival was not improved in most of these patients. This small study suggests that the kinetics of platelet destruction may be improved by intravenous immunoglobulin to an extent to permit the performance of invasive procedures. When all conventional methods fail to increase the platelet count to hemostatic levels, an alternative is continuous platelet transfusion. Although this approach does not result in an increased platelet count, transfused platelets may permit platelet plug formation or help to maintain endothelial integrity, but clinical trial has not tested this assumption.
Prevention of Alloimmunization: Animal studies show that depletion of contaminating leukocytes from donor blood components is effective in prevention of alloantibody response to major histocompatibility complex.
36 In the Trial for the Reduction of Alloimmunization to Platelets, 534 leukemic patients receiving induction chemotherapy were studied during an 8-week period for the development of platelet alloantibodies. HLA antibodies developed in 45% of patients in the control group, compared to 18% in the filtered platelet concentrate group and 17% in the filtered apheresis group.
37 Not all patient groups may benefit from leukoreduction, for example, cardiac surgery patients who are not immunosuppressed may still develop HLA antibodies even with leukoreduced platelet transfusions.