Pituitary Gland Cancer
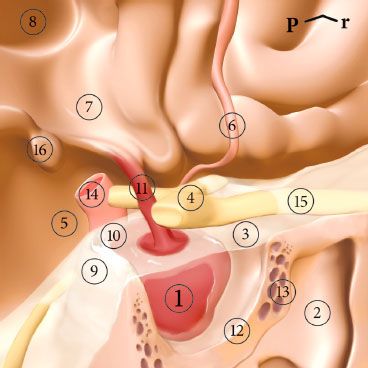
FIGURE 36.1. Posterolateral view of the pituitary gland. P, posterior; r, right. 1, pituitary gland; 2, sphenoid sinus; 3, diaphragm sellae; 4, optic chiasm; 5, chiasmatic cistern; 6, anterior cerebral artery; 7, hypothalamus; 8, third ventricle; 9, dorsum sellae; 10, posterior clinoid; 11, pituitary stalk; 12, sella turcica; 13, cavernous sinus; 14, internal carotid artery; 15, right optic nerve; 16, mamillary body.
 ANATOMIC CONSIDERATIONS
ANATOMIC CONSIDERATIONS
The pituitary gland, also known as the hypophysis cerebri, is an important endocrine organ. It is an ovoid body, the main portion of which is situated in the hypophysial fossa of the sphenoid bone (Fig. 36.1). The main portion is connected to the brain by the infundibulum. The diaphragma sellae forms a dural roof for the greater part of the hypophysis and is pierced by the infundibulum. In front of the infundibulum, the superior aspect of the gland is related directly to the arachnoid and pia1 and the subarachnoid space then extends below the diaphragma.2 The gland is surrounded in the fossa by a fibrous capsule that fuses with the endosteum.3
The hypophysis is related above to the optic chiasma and below to the intercavernous venous sinus and the sphenoid air sinus, allowing an endonasal approach for surgical purposes4 and laterally to the cavernous sinuses. By causing pressure on the chiasma, hypophysial tumors commonly result in visual defects such as superior temporal anopsia or bilateral temporal hemianopsia.
The hypophysis is best divided on embryological bases into two main parts5: the adenohypophysis and the neurohypophysis. The former comprises the pars infundibularis (or pars tuberalis), the pars intermedia, and the pars distalis. The latter comprises the median eminence, the infundibular stem, and the infundibular process or neural lobe. The median eminence is frequently also classified as part of the tuber cinereum. The term infundibulum or neural stalk is used for the median eminence and the infundibular stem. The term hypophysial stalk usually refers to the pars infundibularis and the infundibulum.
The adenohypophysis constitutes about 80% of the total volume of the pituitary gland6 and is a diverticulum of the buccopharyngeal region. The pars distalis area secretes a number of hormones. The neurohypophysis develops as a diverticulum from the floor of the third ventricle. Specifically, it is not an actual endocrine-producing gland but more appropriately should be considered a storage gland for neurosecretions produced by the hypothalamus, which are then carried down via the axona of the supraopticohypophysial tracts.
Blood Supply and Innervations
The hypophysis is supplied by a series of hypophysial arteries from the internal carotids. The maintenance and regulation of the activity of the adenohypophysis are dependent on the blood supply by way of the hypophysial portal system.7–11 Nerve fibers from the hypothalamus liberate releasing factors into the capillary beds in the infundibulum, and these substances are then carried by the portal vessels to the distal parts of the gland, causing the effects relevant to the specific secretions. The neurohypophysis receives its main nerve supply from the hypothalamus by way of fibers known collectively as the hypothalamohypophysial tract. This tract contains two sets of fibers: the supraopticohypophysial tract and the tuberohypophysial tract.
Gross Appearance
Upon gross examination, the pituitary gland is a small, gray, rounded gland that developed from ingrown oral epithelium known as Rathke’s pouch as an extension of the developing oral cavity. Developmentally it is eventually cut off from its origins by the growth of the sphenoid bone and settles into a saddle-shaped, base-of-brain, bone depression called the sella turcica. The anterior pituitary has a portal vascular system that is the conduit for the transport of hypothalamic-releasing hormones from the hypothalamus to the anterior pituitary. Hypothalamic neurons have terminals in the median eminence where the hormones are released into the portal systems. This vascular supply traverses the pituitary stalk and then enters the anterior pituitary lobe. Most pituitary hormones are controlled predominately by releasing factors from the hypothalamus, with the exception of prolactin, which is controlled by the dopamine system via an inhibitory mechanism. It is attached to the lower surface of the hypothalamus by the infundibular stalk. The Rathke’s pouch portion forms the anterior lobe and the intermediate area. The posterior pituitary is embryologically derived from an out-pouching from the floor of the third ventricle and grows inferiorly along the stalk of the anterior lobe. In contrast to the anterior lobe, this posterior lobe is supplied by the inferior hypophyseal artery, which will drain into the venous sinus system to directly release into the systemic circulation. As such, the pituitary gland has a dual circulation; one is composed of arteries and veins, the other a portal venous system that links the hypothalamus and the anterior lobe. The neural tissue of the infundibular stalk forms the posterior lobe. In general, the pituitary gland averages 1.3 é 1.0 é 0.5 cm in size and weighs 0.55 to 0.6 g but enlarges during pregnancy.12 Overall, it is about the smallest functioning gland in the human body, having an important role in physiologic regulation.6
Physiology
There are five cell types that are revealed using specific antibody staining:
1. Somatotrophs. Growth hormone–producing acidophilic cells constituting about 50% of the anterior lobe.
2. Lactotrophs. Prolactin producing acidophic cells (also known as mammotrophs).
3. Corticotrophs. Basophilic-appearing cells that produce adrenocortitrophic hormone, pro-opiomelanocortin, melanocytic-stimulating hormone, endorphins, and lipotropin.
4. Thyrotrophs. Very pale appearing cells that produce thyroid-stimulating hormone.
5. Gonadotrophs. Basophilic cells that produce follicle-stimulating hormone and luteinizing hormone.
 MORPHOLOGY
MORPHOLOGY
The most common pituitary adenoma is a soft, well-circumscribed lesion that may be confined to the sella turcica. Larger lesions typically extend superiorly through the diaphragm sella into the suprasellar region. This can cause compression of the optic chiasm and adjacent structures, including cranial nerves. Continued expansion eventually erodes the sella turcica, the anterior clinoid process, and even into the cavernous and sphenoid sinuses. As many as 30% of cases adenomas can be nonencapsulated and infiltrate adjacent bones, dura, and the brain proper, although rarely.13 Foci of hemorrhage and necrosis are hallmarks of these invasive larger adenomas. Pituitary apoplexy occurs when an acute hemorrhage causes a rapidly enlarging mass, resulting in sudden onset of mass effect symptoms.
 CLINICAL COURSE
CLINICAL COURSE
The signs and symptoms of pituitary adenomas include endocrine abnormalities and mass effects. Abnormalities associated with excessive secretions of anterior pituitary hormones are specific to the particular aberrant cell line, as described below. Local mass effect can be associated with any type of pituitary tumor, including a rare metastatic tumor from another site. The earliest changes in local anatomy result in radiographic changes with local deformation of the sella, the diaphragm, and bone erosions. Ultimately, compression of surrounding normal soft tissues results in visual field abnormalities with headache, nausea, and vomiting; eventually compressing the nonneoplastic pituitary and resulting in hypopituitarism. This can occur rapidly if there is hemorrhage resulting in pituitary apoplexy with excruciating headache, diplopia from pressure on the oculomotor nerves, and hypopituitarism.
The posterior pituitary or neurohypophysis consists of pituicytes—modified glial cells—and axonal processes extending from nerve cell bodies in the supraoptic and paraventricular nuclei of the hypothalamus. These cells transit through the pituitary stalk to the posterior lobe where the two posterior lobe hormones, oxytocin and vasopressin, are stored.
Hormones are secreted in the following lobes:
• Intermediate lobe: no effects are known in warm-blooded mammals.
• Anterior lobe: Growth hormone–regulating cell division and protein synthesis; adrenocorticotrophic hormone, which regulates the functional activity of the adrenal cortex; thyroid-stimulating hormone, which regulates the functional activity of the thyroid gland; follicle-stimulating hormone, which regulates ovarian follicles and spermatogenesis; luteinizing hormone (in women), which stimulates ovulation, formation of the corpus luteum, and secretion of estrogen and progesterone. In men, it can be called interstitial cell–stimulating hormone, which stimulates testosterone secretion. Also in women there is prolactin, which induces secretion of breast milk.
• Posterior lobe: Hormones are secreted by the neurosecretory cells of the hypothalamus and pass through the fibers of the supraopticohypophyseal tracts in the infundibular stalk to the neurohypophysis where they are stored. These stored secretions are oxytocin, which acts on smooth muscles of the female uterus to increase contractility. There is also antidiuretic hormone, which increases water reabsorption of the renal system via the kidney tubules, and a derivative known as vasopressin to regulate blood pressure.
 DISORDERS
DISORDERS
Diseases of the pituitary are divided into those that affect the anterior lobe and those that affect the posterior lobe. The former are either hypersecretory or hyposecretory in nature. In most cases, hypersecretion is caused by a functioning adenoma within the anterior lobe. Hypopituitarism may be caused by a variety of destructive processes, including ischemic injury, radiation exposure (including therapeutic radiation), inflammatory responses, and nonfunctioning tumors such as a squamous “pearl” from cell rests that occurs during embryo development. Other than endocrine abnormalities, diseases of the anterior pituitary may manifest by a local mass effect. Radiographically this can usually be seen by enlargement of the sella turcica, clinically by visual field defects or visual cuts, and evidence of increased intracranial pressure by optic examination or a patient’s complaint of headache and nausea. The posterior pituitary may provide clinical evidence of antidiuretic hormone abnormalities.
Hyperpituitarism and Pituitary Anterior Adenomas
Excess production of hormones related to the anterior pituitary is often caused by a benign adenoma arising from the anterior lobe. Less commonly, there can be hyperplasia or, rarely, actual carcinoma of glandular elements. Interestingly nonfunctioning adenomas may cause hypopituitarism as they grow and displace the normal functioning gland. Functional pituitary adenomas are usually composed of a single aberrant cell type, thus producing a single dominant hormone. Less common are some adenomas that may have a single cell line but produce more than one hormone product. Only rarely are adenomas with multiple cell lines. The vast majority of pituitary adenomas are monoclonal in origin, suggesting a single somatic cell, even when a plurihormonal diagnosis is made.14 Some plurihormonal adenomas may arise from a primitive stem cell, which may subsequently differentiate to different productive cell lines simultaneously. Molecular studies have identified specific mutations, such as a single base-pair missense mutation that would stabilize one protein into an active formation while inhibiting a controlling protein, thus mimicking an active hormone. This has been identified in about 40% of growth hormone–secreting tumors. Other molecular aberrations seem more sporadic, and the pathogenesis of most pituitary tumors is still largely unknown.
 PHYSIOLOGY
PHYSIOLOGY
Hyperpituitarism of the Pituitary Lobes
Hypersecretion of the anterior lobe causes gigantism, acromegaly, and Cushing disease (pituitary basophilism). Hyposecretion of the anterior lobe causes dwarfism, Simond disease (pituitary cachexia), postpartum pituitary necrosis of Sheehan syndrome, acromicria, eunuchoidism, and hypogonadism. A posterior lobe deficiency is a hypothalamic lesion that causes diabetes insipidus. Anterior and posterior lobe deficiencies plus a hypothalamic lesion causes Frohlich syndrome (adiposogenital dystrophy) and pituitary obesity.
Box 36.1
Pituitary Adenoma Management Overview
Symptomatic adenomas present for medical attention as a result of hormone secretions, compression of nearby normal structures with neurologic symptoms, or compression of the pituitary stalk causing hypopituitarism. An initial therapy for most prolactinomas is with a dopamine agonist such as bromocriptine, lysuride, or pergolide. Medical intervention usually decreases adenoma function and size. Initial therapy for other pituitary adenomas is transsphenoidal surgical resection. Surgery is generally safe and reverses neurologic symptoms, with most patients normalizing hormone levels. It is mostly useful to cure microadenomas. Radiation therapy is often reserved for patients with residual disease after surgery, such as after a debulking surgery. It is also considered for recurrence after definitive surgery or for medically inoperable patients. Typically, conventional radiation delivers a dose of 45 Gy at 1.8 Gy daily fractions. At this dose, good control can usually be achieved with a very low risk of optic neuropathy. Normalization of hormone levels, however, can take months to years to achieve.
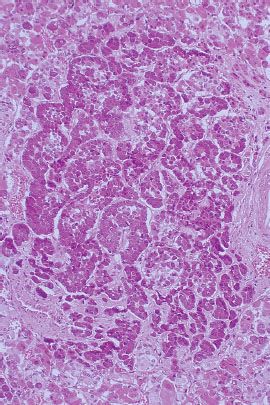
FIGURE 36.2. Photomicrograph of microadenoma. (From Damjanov I. Histopathology: a color atlas and textbook. Baltimore: Williams & Wilkins, 1996, with permission.)