Fig. 2.1
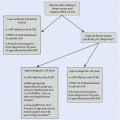
a The milk lines in a generalized mammalian embryo. Mammary glands form along these lines. b Common sites of formation of supernumerary nipples or mammary glands along the course of the milk line in human (Reproduced from Skandalakis et al. [22]; with permission)
At 16 mm fetal length, the mammary band has disappeared, while the breast primordium has almost sunk inside the mesoderm, while at 55 mm fetal length, the primitive nipple appears as a well-circumscribed mass of cells at the apex of an elevation. Nipple formation is almost complete at 90 mm fetal length with keratinization of the superficial cells of the mammary bud. Superficial cells are easily discernible since, in contrast to periderm, they are CK14 negative [5].
At this point, the first stage of fetal breast development is complete. At 170 mm fetal length, the primordium of the ducts appears as solid cellular outgrowths that arise from the deep surface of the mammary bud. They pierce the first two layers of the mesoderm, without being invested by them and receive a covering from the third layer [7]. Transformation of these solid outgrowths into lobules occurs through desquamation and lysis of the central cells. Ducts are further branched and acquire several terminal branches. At this stage, at 300 mm fetal length, two discrete cell populations are seen inside the ducts: luminal and myoepithelial. Separation into lobes has already begun with fibrous septa originating from the fourth layer of the mesoderm [9]. At 330 mm fetal length, the development of the fetal breast is almost complete with completion of canalization of the tubules and their organization into groups of ducts separated into lobules. Lobules are covered by two cell layers, one cuboidal and one flattened. The zones of the mesoderm have completed their differentiation. Dense vascular plexuses at the end of the ductal structures have appeared and drain into vascular lakes in the second layer of the mesoderm. The mammary gland is enclosed in a thin capsule of connective tissue with a similar appearance to a series of small sebaceous gland related to the mammary ducts. No hair follicles are seen at the nipple. At this point male and female mammary glands are not discernibly different.
2.2.2 Congenital Anomalies
Most congenital anomalies and variations originate during this prenatal period of development. The ulnar-mammary syndrome that manifests as aplasia or hypoplasia of the breast and anomalies of the ipsilateral limb is attributed to absence of the T-box transcription factor (TBX3) gene [5].
Isolated amastia or hypoplasia of the breast without other pectoral defects is rarer, and athelia (congenital absence of the nipple only) without amastia is an extremely rare situation. No responsible genes for the aforementioned conditions have been found.
Accessory nipples and accessory breasts are a relatively common phenomenon, with an incidence of 1–5%. They develop along the mammary line and require no treatment except if they become symptomatic, usually during lactation. European populations usually present with supernumerary nipples below the normal nipple and Japanese populations usually above. Juvenile or virginal hypertrophy of the breast and fibroadenomas are better explained as anomalies associated with breast development and are not correlated with any hormonal abnormality.
Gynecomastia is an acquired rather than a congenital situation. It is often caused by drugs or by oestrogen regulation in cirrhotic patients. Gynecomastia is secondary to ductal and stromal development but not to lobular development due to the immaturity of the male breast [5, 8, 10, 11].
Deformities of the thoracic wall are often accompanied by deformities of the breast. They are often explained as secondary to rib morphogenesis abnormalities. They should be treated in an individualized manner, with respect to patient’s other abnormalities, symptoms and life expectancy. Commoner are a depressed chest (pectus excavatum), protruding chest (pectus carinatum/pigeon breast) and mixed deformities [10]. The most well-known syndrome is Poland’s syndrome, characterized by:
Absence of costal cartilages and a portion of the third or third and fourth rib
Absence of the nipple or breast with accompanying hypoplasia
Absence of subcutaneous fat
Absence of axillary hair
Absence of the pectoralis minor muscle
Absence of costosternal part of the pectoralis major muscle [8]
2.2.3 Infant Breast
During intrauterine life, the fetal breast is under hormonal control of oestrogen and progesterone produced by the placenta. Oestrogens promote tissue development, growth of the network of breast ducts and fat deposition, while progesterone promotes lobuloalveolar differentiation. Oestrogen receptors are found to be expressed in the infant breast tissue from birth to 2–3 months of age [12]. Oestrogen and progesterone are inhibitors of prolactin synthesis. Immediately after birth, fetal prolactin increases temporarily which leads to breast development and milk production, commonly referred to as «witch’s milk» [13, 14].
Soon after birth, the breast involutes rapidly and remains as such until puberty. Until puberty, the only change is ductal elongation in accordance with body size. There is no further maturation [15]. Soon after birth, the nipple everts due to an increase in stroma, and areola pigmentation increases [16]. The infant breast contains lobular structures, dilated ducts, apocrine secretions, extra-medullary haematopoiesis and the presence of myoepithelial cells [17].
McKiernan and Hull [18] investigated the infant breast and stated that most infants have palpable breasts and are capable of milk production on gentle palpation. They also stated that breast development corresponds to gestational age and not body size, and thus breasts are not usually palpable in preterm infants.
Anbazhagan and colleagues [19], taking the above considerations into account, stated that the infant breast can be categorized into three different morphologic types:
Type I: Rudimentary ductal system, single elongated ducts, no branching or less than two dichotomous branchings and two layers of epithelium
Type II: More than two dichotomous branchings, no terminal lobules and few budlike projections lined by stratified epithelium
Type III: Branching ductal system and developed terminal lobules
They also classified functional status into five different grades:
Type I: Whole ductal system lined by luminal cells, single layer of myoepithelial cells and loose intralobular connective tissue
Type II: Cystic dilation of the ducts. Secretory- and apocrine-type epithelium
Type III: Most ductal system shows cystic dilation. Existence of grossly dilated lobules and apocrine metaplasia
Type V: A complete involuted gland
Type IV represents a transition between types III and V
According to these authors, [20] these differences might be caused by different levels of maternal oestrogen during intrauterine life.
2.2.4 Peripubertal Breast
Soon after birth, low GnRH levels and consequently low FSH and LH levels lead to low oestrogen and progesterone levels. The breast therefore remains inactive until puberty [15].
Probably due to secondary mediators, breast development restarts before levels of sex hormones can be discriminated between the sexes [11]. At adrenarche, an increase of androgens and pregnenolone restarts breast maturation. In men a transient breast enlargement might become clinically important, yet it will soon involute [15]. In women, menarche signifies oestrogen production that, in cooperation with androgens, will continue to stimulate breast development. Amphiregulin, human growth factor, epidermal growth factor and insulin growth factor have all been proposed as paracrine mediators of oestrogen effects. Microscopically, the ducts become elongated, the epithelium becomes thicker and the stromal tissue increases. Immature ducts undergo branching and extend into underlying tissue giving rise to a segmental pattern. Subsegmental and terminal ducts eventually give rise to acini. Acini from a single terminal duct surrounded by fibrous tissue constitute the functional unit of the breast: the terminal duct lobular unit (TDLU). Four types of lobule are recognized: Type 1 comprise short terminal ducts ending in a cluster of secretory cells, the alveoli. Types 2 and 3 comprise ducts ranching into several ductules and an increasing number of alveoli. Type 4 is seen in women that have gone through pregnancy and lactation and will be discussed later [16].
By age 15, the central breast is shaped. After that, and until the first pregnancy, it expands peripherally acquiring the form of the nulliparous adult breast [5, 11, 13, 15]. Until the first pregnancy, the breast is under the hormonal control of the menstrual cycle. During the menstrual cycle, the breast size increases in the luteal phase due to stromal swelling rather than further duct development. Yet functional changes have been observed through an increase in mitoses and apoptosis during the luteal phase of the cycle [15]. Notably in a mature breast, the glandular component constitutes less than 20% of the whole breast.
Macroscopically, Marshall and Tanner [21] classified the prepubertal breast into five categories (◘ Fig. 2.2):
First (prepubertal): Elevation of the papilla
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree