Phagocytosis and Intracellular Killing
Learning Objectives
• Restate the role of precipitin reaction in phagocytosis
• Define the zone of equivalence
• Identify the three different antibody Fc receptors in phagocytic cell membranes
• Compare and contrast pinocytosis and phagocytosis
• Explain the respiratory burst
• Identify the role of superoxide dismutase in the generation of hydrogen peroxide (H2O2)
• Define the reactive oxygen species
• Identify the role of myeloperoxidase in intracellular killing
• Recognize the constituents of primary, secondary, and tertiary granules
• Explain the relationship between chronic inflammation and cancer
• Understand the alternative mechanism for generating mutagenic nitrosoamines or N-nitrosoamides
• Recognize the immunologic defect in Gaucher’s disease
• Design a therapeutic regimen to treat Gaucher’s disease
• Recognize the immunologic defect in chronic granulomatous disease (CGD)
• Analyze the importance of cytochrome b588 in respiratory burst
• Design acceptable treatment modalities for CGD
• Identify the fungal species that commonly infect patients with myeloperoxidase (MPO) deficiency
• Explain the relationship between CR3 and leukocyte adhesion deficiency type I
Key Terms
Catalase
Chronic granulomatous disease
Gaucher’s disease
Myeloperoxidase
Oxygen-dependent intracellular killing
Oxygen-independent intracellular killing
Peroxidase
Phagocytosis
Pinocytosis
Reactive oxygen species
Respiratory burst
Singlet oxygen
Superoxide dismutase
Introduction
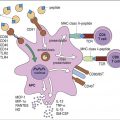
Phagocytosis and intracellular killing comprise the final step in the resolution of extracellular microbial infections. Phagocytic cells express receptors for the Fc domain of bound antibody and opsonizing complement fragments. After binding to the receptors, antigens or intact microbes are ingested and destroyed by oxygen-dependent and oxygen-independent killing mechanisms. As a prelude to phagocytosis, microbes are rendered insoluble by the chemical properties of the antigen–antibody complexes and the action of anaphylatoxins.
Precipitation of Antigen–Antibody Complexes
Phagocytosis is more efficient when antigen–antibody complexes are insoluble. The molar concentration of antigens and antibodies in plasma determines immune complex solubility. In the early phase of the immune response to the foreign protein, few antibodies are directed at antigens, and the very small immune complexes can pass through the kidney. As the concentration of antibodies increases, the ratio of antibodies to antigens decreases, and very large immune complexes are formed. When equimolar concentrations of antigens and antibodies are present (the equivalence zone), large complexes are deposited in small capillaries and in the renal glomeruli. Deposition of immune complexes is augmented by anaphylatoxins released during complement activation.
Cellular Receptors for Complement
CR1 Complement Receptor
The CR1 complement receptor (CD35) is expressed on a number of blood cells, including polymorphonuclear leukocytes, monocytes or macrophages, and follicular dendritic cells. These receptors are single-chain membrane proteins with multiple short consensus receptors (SCRs). Binding of complement fragments occurs some distance from the cell. The CR1 complement receptor recognizes C3b, iC3b, and C4b.
CR2 Complement Receptor (CD21)
The CR2 membrane glycoprotein receptor (CD21) binds the complement decay fragments iC3b and C3dg. CR2 is expressed by follicular dendritic cells in the germinal centers and serves to trap antigen–antibody complexes on iccosomes.
CR3 Complement Receptor (Mac-1, CD11b/CD18)
Complement receptor type 3 (CR3, Mac-1, and CD11b/CD18) is found on monocytes, neutrophils, natural killer (NK) cells, and dendritic cells. CR3 is composed of α and β chains. The CD11b receptor interacts with cell-bound iC3b to initiate phagocytosis. CD18 forms the β2-chain of CD11a, CD11b, and CD11c (leukocyte adhesion molecule). CD18 is the major component of lymphocyte function–associated antigen 1 (LFA-1), macrophage antigen 1 (Mac-1), and CD4 integrins, which bind leukocytes to the capillary endothelium.
Fc Receptors for Immunoglobulin G
Three different immunoglobulin G (IgG) receptors (CD64, CD16, and CD32) are expressed on phagocytic cells. Fc-gamma receptor I (FcγRI or CD64) is a high-affinity, isotype-restricted receptor that is present on most phagocytic cells, eosinophils, and dendritic cells. CD16 (Fc receptors FcγRIIIa and FcγRIIIb) is a low-affinity Fc receptor expressed on neutrophils, NK cells, eosinophils, and macrophages. The CD32 receptor is expressed on B lymphocytes and cells of macrophage or monocyte lineage. It is also known as a low-affinity Fc-gamma receptor II (FcγRII), which binds immune complexes.
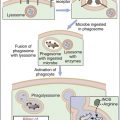
Ingestion of Antigens or Microbes
Endocytosis is a general term describing a process by which cells absorb external material by engulfing it with the cell membrane. Endocytosis is usually subdivided into pinocytosis and phagocytosis. If the antigen is a small-molecular-weight protein or a polysaccharide, the cell membrane invaginates in a process called pinocytosis, and the protein or carbohydrate is placed in a fluid-filled sack called a vesicle. Large-molecular-weight antigens or intact microbes are internalized by a different form of endocytosis, which is called phagocytosis. During phagocytosis, the membrane envelopes the particle to form an internal vacuole termed phagosome.
Intracellular Killing of Bacteria
In the phagosome, bacteria are killed by two different mechanisms. One mechanism is dependent on the presence of oxygen, a respiratory burst, and the generation of reactive oxygen species. The second mechanism (which is independent of oxygen) uses preformed granules containing proteolytic enzymes to kill microbes.
Oxygen-Dependent Respiratory Burst
Following the ingestion of microbes, an oxygen-dependent respiratory burst occurs, with rapid production of singlet oxygen ( ) and hydrogen peroxide (H2O2) and energy in the form of adenosine triphosphate (ATP). In the oxidative phosphorylation pathway, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase generates singlet oxygen as it transfers electrons to the cytochrome system.
) and hydrogen peroxide (H2O2) and energy in the form of adenosine triphosphate (ATP). In the oxidative phosphorylation pathway, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase generates singlet oxygen as it transfers electrons to the cytochrome system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree