Setting
Study [Reference]
No.
Residual disease
Survival
Primary cytoreduction
5-year (%)
Median (months)
Hoskins (1994) [5]
41
R 0
60
62
≤1 cm
35
77
>1 cm
20–35
Chi (2006) [6]
67
R 0
106
70
≤ 0,5 cm
66
328
> 0,5 cm
33–48
du Bois (2010) [40]
1,046
R 0
99.1
975
≤ 1 cm
36.2
1,105
> 1 cm
29.6
Secondary cytoreduction
Authorsa
513
R 0
30–63.2
441
Any residual
7.2–27.6
More controversial is the role of CRS in the setting of recurrent disease in which the prognosis is related to a large number of factors, such as patient age, interval between initial diagnosis and relapse, presence of ascites, and histologic subtype. Even in the absence of prospective studies, multiple retrospective studies [8–17] (Table 20.1) and a meta-analysis involving 40 studies and 2,019 patients [18] confirmed the prognostic role of maximal cytoreduction in recurrent cases. Even after tertiary and quaternary cytoreduction, total cancer removal is a reliable prognostic factor, as constantly demonstrated in relevant publications, although this assumption is based on the analysis of retrospective studies only [19–22].
Tumor reduction surgery has evident benefits: it improves the patient’s QoL, enhances tumor susceptibility to CHT by stimulating the active division of cells, and decreasing the likelihood of drug-resistant clones by removing gross necrotic tumor masses. Maximum cytoreduction able to permit complete removal of peritoneal disease is therefore the most significant prognostic factor in all settings and currently represents the fundamental aim of surgery when this approach is indicated.
20.2.1.1 Peritonectomy
PC is characterized by neoplastic involvement of both the parietal and visceral peritoneum, and radical surgery involves removing the peritoneal areas affected by carcinomatosis.
In treating ovarian carcinomatosis, debulking, cytoreduction, and PRT— even if considered as synonyms of the same concept, i.e. maximal removal of cancerous tissue—express the progressive evolution toward more extensive surgery and match criteria and techniques described in Chap. 9. All endoperitoneal viscera and parenchyma are susceptible to partial or total excision, according to the degree of invasiveness and the feasibility of in situ cytoreduction of implants, as previously described (Fig. 20.1). However, some aspects need further clarification, as they are related to visceral resection and lymphadenectomy.
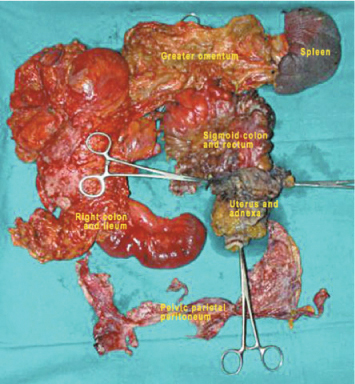

Fig. 20.1
Visceral and parietal peritonectomy for ovarian carcinomatosis
Until now, type and extent of visceral resection for locally advanced EOC have reflected the different attitudes of key figures dealing with these procedures; there are clear-cut differences between the approach of oncologic surgeons engaged in PSM treatment and that of gynecologic oncologists. The aim of the surgeon performing PRT is to achieve complete cytoreduction (CC-0) or optimal CRS with residues up to 2.5 mm, whereas gynecologic oncologists— with few exceptions—consider that optimal cytoreduction is achieved even when residual disease includes nodules of 1 or 2 cm. Such limits in many cases can result in residual disease classification of FIGO stage III, the same stage as before cytoreduction. This low aggressive approach toward eradicating the disease is adopted especially when the carcinomatosis involves critical areas difficult to treat, such as the supramesocolic space or when there is an extensive infiltration of the colon and rectum. Such cases of partial cytoreduction are assigned to subsequent chemotherapies that are often carried out by the same gynecologists, with the aim being more toward disease chronicity rather than treatment radicalization. Recently, even oncologic gynecologists have begun recognizing the importance of maximal cytoreduction in the supramesocolic space, but it must be noted that few gynecologic centers have sufficient surgical skills to address this technical challenge [23].
Particularly relevant is the problem of PC treatment when it involves the colon and the rectum, as discussed in specific studies [24, 25] (Fig. 20.2). Widespread pelvic disease, with involvement of the pouch and colorectal wall, entails resection of this viscus with the same criteria of radicality adopted when treating primary CRC (Colorectal Cancer), i.e., including mesorectal excision and sections of mesenteric vessels at their origin. Similar radical colonic resections should be performed when other large-bowel sectors are involved (Fig. 20.3). Using this procedure allows both removal of a large amount of mesocolon—frequently infiltrated by implants—and regional lymphadenectomy that removes lymph node stations, which are metastatic in > 50 % of cases [24, 26]. In primary CRS, pelvic and para-aortic lymphadenectomy must be routinely performed in relation to the high incidence of locoregional metastases. In secondary CRS, locoregional lymph node dissection should be performed if not done during primary cytoreduction or if there is evident of lymph node recurrence (Fig. 20.4).
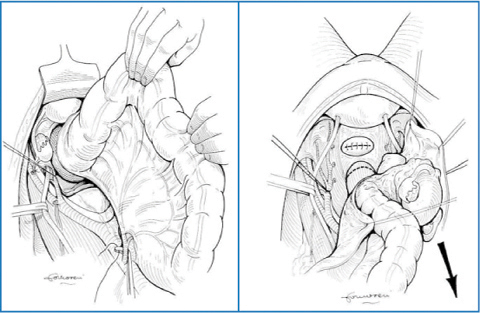
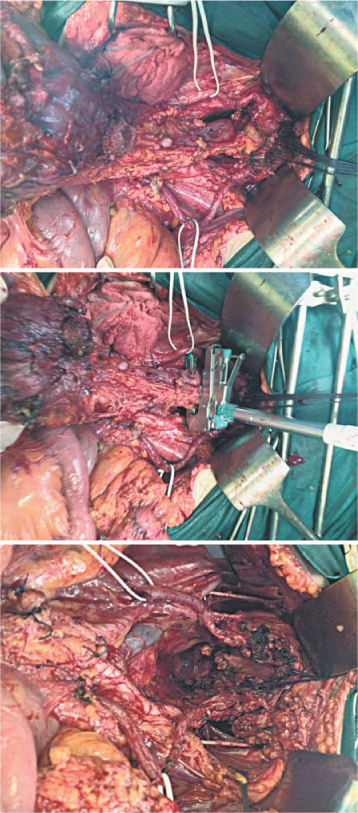
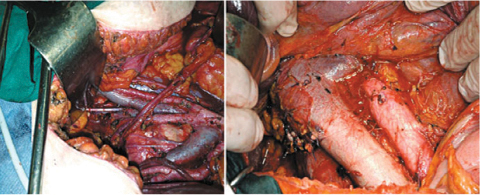

Fig. 20.2
En bloc hysteroadnexectomy, pelvic-parietal peritonectomy, and colorectal resection. Inferior mesenteric artery is ligated at the aortic origin

Fig. 20.3
Pelvic peritonectomy: hysteroadnexectomy, colorectal en-bloc resection, and pelvic lymphadenectomy

Fig. 20.4
Pelvic, para-aortic, and paracaval lymphadenectomy
In patients who undergo NACT and attain a complete or partial response, persistent morphological alterations of the peritoneal membrane identify the sites of previous carcinomatosis, as described in Chap. 4. Accurate evaluation of such morphological alterations is invaluable in planning a correct CRS strategy. If there is no evidence of macroscopic disease, basic hysteroadnexectomy, locoregional lymphadenectomy, complete omentectomy, appendectomy, and resection of the ligamentum teres and falciform ligament should be performed according to criteria described in Chap. 9. In these cases, it is appropriate in principle to perform a parietal PRT of the lower abdomen and pelvis below the transverse umbilical line to remove the peritoneum, which is often involved in carcinomatosis. All other residual peritoneal areas with previously described morphological alterations should be treated superficially and extensively with argon or ball-tip electrosurgery to alter structural continuity of post-CHT fibrosis, thus allowing deeper penetration of drugs into the peritoneal membrane during HIPEC.
20.2.2 Peritonectomy plus Hyperthermic Intraperitoneal Chemotherapy
Removing all macroscopically visible tumor tissue is not an absolute guarantee of neoplastic “sterilization” of the abdominopelvic cavity; persistence of microscopic residual is possible. Therefore, a more suitable means of treatment is required to ensure control over microscopic residual. From this perspective, HIPEC at the end of the surgical phase is a logical method of addressing this problem, as for other forms of PSM. The principles and rationale underlying the association of PRT plus HIPEC, together with results from the largest multiinstitutional case studies, are the major factors supporting the adoption of this integrated procedure in treating primary and recurrent ovarian carcinomatosis.
Cisplatin (CDDP), at doses varying from 50 to 100 mg/m2, is the most widely used drug for HIPEC in ovarian carcinomatosis. The isolated or combined administration of other drugs in association with CDDP is used in some centers. In most centers treating ovarian carcinomatosis with this combined procedure, general criteria for patient inclusion are rather homogeneous and relate to patients without extra-abdominal disease, with optimal American Society of Anesthesiologists (ASA) and performance status (PS) scores and with surgically cytoreducible disease. Patient age is not a matter of absolute exclusion; even patients in their 80s, particularly motivated, with good ASA and PS scores with limited and easily resectable PC can be scheduled for the procedure. The presence of easily resectable and isolated liver metastases is not a contraindication for the procedure if complete cytoreduction is achievable. The role of PCI as a factor conditioning patient inclusion is not uniformly accepted for ovarian carcinomatosis: some authors identify the levels beyond which PRT plus HIPEC is inadvisable [27–29]; for others, limitation is related to the infeasibility of cytoreduction. When the cytoreduction score is suboptimal, HIPEC is theoretically avoidable, as it is not effective in attacking residues > 2.5–3 mm; but if ascites is present, then HIPEC can still be performed, as it is effective in preventing ascites reproduction in a large percentage of cases.
PRT plus HIPEC is applicable in various phases of the disease and in relation to different evolutionary scenarios related to previous treatments. Therefore, four possible treatment settings are predictable:

20.3 Role of Peritonectomy plus Hyperthermic Intraperitoneal Chemotherapy in Treating Peritoneal Carcinomatosis from Ovarian Cancer
Since 2000, the use of HIPEC combined with maximum cytoreduction (PRT) for treating PC from OC has gradually become more widespread. Results obtained thus far, although drawn exclusively from phase 1 and 2 studies of small and inhomogeneous case series, encourage the continuation of experience in this field and continue to support the benefits for long-term survival in both primary and recurrent forms, with acceptable morbidity rates when performed in highvolume HIPEC centers. However, inhomogeneity when selecting patients for neoadjuvant and adjuvant treatments and when assessing and classifying PC spread and cytoreduction levels complicates comparative data analysis and opens up the results of multicenter studies to criticism. Moreover PRT requires a high number of surgical resections to eradicate a disease with multiform diffusion, in the face of which surgical attitude is hardly comparable, so that the surgeon is a prognostic factor of considerable significance [30]. Furthermore, the risk of high morbidity and lack of controlled studies further exacerbate this general scepticism, particular regarding the role of HIPEC.
At present—and pending results of future prospective trials—the role and limitations of applying the procedure are drawn from experiences from three basic study types (Table 20.2):
Collective reviews
Multicenter studies
Monocentric case studies produced by high-volume HIPEC centers
Table 20.2
Peritonectomy plus HIPEC for treating ovarian carcitomatosis: literature review and ongoing studies (2,419 cases)
Type of study | Year | Study [Reference] | Study design | No. cases | No. studies/centers |
|---|---|---|---|---|---|
Collective reviews | 2012 | de Bree and Helm [33] | Collection of phase II | 1,102 | 22 |
Multicenter | 2010 | Helm et al. [34] (HYPER-O) | Retrospective | 141 | 9 |
2011 | Deraco et al. [35] | Prospective phase II | 26 | 4 | |
2013 | Bakrin et al. [29] | Retrospective | 566 | 13 | |
2014 (unpublished) | De Iaco, Multi Institutional Italian Study (MIIS)* | Prospective phase II | 454 | 13 | |
Monocentric | 2014 unpublished | Di Giorgio | Prospective phase II | 130 | 1 |
20.3.1 Collective Reviews
The most important available collective reviews are the studies by Bijelic et al., Chua et al., and de Bree [31–33]. In their (more recent) review, de Bree and Helm present results of 22 studies, 12 and 9 of which are also considered by Chua et al. and Bijelic et al. in their collections. These collective studies report data from phase I and II studies that are remarkably heterogeneous, particularly in terms of drugs used in HIPEC, post-HIPEC results, and duration of followup. The reviews are primarily focused on evaluating survival, morbidity, and postoperative mortality rates but summarize the other results of the individual studies partially and rather inhomogeneously. Concerning survival, base parameters that impact prognosis, such as CC and PCI scores, are either not analyzed or are only marginally considered: concerning morbidity, common risk classifications are not evaluated. Even if with some inaccuracy on data retrieval that have been rectified or not taken into consideration in this analysis, the de Bree/Helm review can be considered the most complete and to best represent current results inferable from collective reviews.
20.3.2 Multicenter Studies
Multicenter studies are the most frequently reported in the literature, but similarly to reviews, they show such a high degree of heterogeneity both in methods and in contents that comparison between them is often difficult. Two studies are retrospective: the first [34] refers to the Hyperthermic Intraperitoneal Chemotherapy In Ovarian Cancer (HYPER-O) US register, which refers to seven centers; the second [29] is currently the major collective clinical report and collects data derived from 13 French centers, including 246 cases already reported in a previous work [28]. Two further studies are considered as prospective by their own authors: the Multi-Institutional Italian Study (MIIS), and Deraco et al.’s Multi-Institutional Study [35]. The Italian study is ongoing, and results reported here are as yet unpublished; to date, that study comprises 454 cases from 13 centers, including 109 cases from clinical records of the senior author of this chapter. The primary aim of all these studies—except Deraco et al.’s, which exclusively analyzes front-line-treated cases—is to evaluate longterm survival and morbidity and consider the various settings in which the PRT plus HIPEC procedure is administered. Survival is analyzed with uniform statistical methodology, and in all studies, several types of prognostic factors are considered in univariate and multivariate analysis—except in Deraco et al.’s study, which exclusively and generically analyzes overall (OS) and progression-free (PFS) survival. Among prognostic factors, PCI and CC scores are the most frequently considered. Heterogeneity is evident when morbidity is analyzed, with the majority of reports referring generically to major complications that are classified with inhomogeneous protocols. Few studies analyze risk factors for complication with univariate or multivariate analyses.
20.3.3 Monocentric Study: Personal Experience
Most relevant single-center studies have been included in the reviews described above and summarized in de Bree and Helm’s review [33], but all examine a limited number of cases, with the largest study analyzing 81 patients. The single-center clinical study reported here and until now unpublished represents the largest monocentric study on treating PC from OC with PRT plus HIPEC: 130 cases of PC derived from high-grade and FIGO stages IIIc/IV OC are enrolled in this nonrandomized prospective phase II study on treating ovarian carcinomatosis with PRT plus HIPEC. The study analyzes clinical records of treatment performed between November 2000 and December 2013 by a single surgeon as main operator (Di Giorgio) and assisted by the same surgical and anesthesiological staff. The same team of pathologists analyzed surgical samples following a specific protocol implemented at the beginning of the study. The same radiological staff carried out investigations and reviewed morphological CT and MR images both during the preoperative and follow-up phases. The database was organized prospectively. At the beginning of 2013, part of this series was included in the MIIS described above.
20.4 Study Results
Clinical and anatomopathological characteristics and HIPEC techniques inferable from the analysed studies are summarized in Table 20.3.
Table 20.3
Patient characteristics and hyperthermic intraperitoneal chemotherapy (HIPEC) techniques
Study [Reference] | HYPER-O [34] | Deraco et al. [35] | Bakrin [29] | MIIS (unpublished) | Di Giorgio (unpublished) | de Bree and Helm [33] |
|---|---|---|---|---|---|---|
No. cases | 141 | 26 | 566 | 454 | 130 | 1,102 |
Grade (%) | ||||||
1 | 9.9 | NR | ||||
2 | 8.5 | 15.4 | 8.7 | NR | ||
3 | 74.5 | 84.6 | 85.8 | NR | ||
Undetermined or consolidation | 7.1 | 5.5 | NR | |||
FIGO (%) | ||||||
0 (Consolidation or undetermined) | 2.8 | 5.4 | NR | |||
II | 7.1 | 2.3 | NR | |||
III | 80.1 | 96.2 | 85.4 | NR | ||
IV | 10 | 3.8 | 6.9 | NR | ||
Liver metastasis (%) | 5.5 | 11.9 | 3.1 | NR | ||
Lymph node metastasis (%) | ||||||
Yes | 26.2 | 52.6 | ||||
No | 73.8 | 47.4 | ||||
Setting (%) | ||||||
Frontline | 18.5 | 100 | 2.1 | 9.5 | 17.7 | 18.4 |
Interval debulking | 13.6 | 4.2 | 23.3 | 29.2 | 5.6 | |
Consolidation | 8.6 | 9.9 | 8.8 | 5.4 | 8.9 | |
Recurrence | 59.3 | 83.8 | 58.4 | 47.7 | 67.1 | |
Platinum response (%) | ||||||
Resistant | 34 | 52.1 | 35 | 36.8 | NR | |
Sensitive | 53.9 | 47 | 34.4 | 53.8 | NR | |
Undetermined | 12.1 | 0.9 | 30.6 | 9.4 | NR | |
PCI median | 15.5 (5–26) | 10.6 (0–31) | 10.9 (0–39) | 16.3 (0–39) | NR | |
CC score (%) | ||||||
0 | 58.3 | 57.7 | 74.9 | 71.6 | 66.7 | NR |
1 | 15.1 | 42.3 | 17.9 | 22.3 | 20 | NR |
> 1 | 26.6 | 7.2 | 6.1 | 13.3 | NR | |
HIPEC drugs (%) | ||||||
CDDP | 37.2* | a | 41 | 17.4 | 100 | NR |
Oxaliplatin | 21.3 | 11.5 | NR | |||
MMC | 38.7* | 2.1 | 0.3 | NR | ||
CarboTaxol | 14.6* | – | NR | |||
Doxorubicin | b | 0.2 | – | NR | ||
Adriamycin | 4.2 | |||||
Combination (≥2) | 9.5* | 100 (a +b) | 35.4 | 66.6 | NR | |
HIPEC technique (%) | ||||||
Open | 12.1 | 68.4 | 41.3 | NR | ||
Closed | 87.9 | 100 | 31.6 | 50.2 | 100 | NR |
Semiopen | 9.5 | |||||
HIPEC duration | ||||||
60 min | 64.1 | 100 | ||||
60–90 min | 45.4 | 100 | 35.9 | |||
90–120 min | 546 | – | ||||
Adjuvant CHT (%) | ||||||
Yes | 93.6? | 100 | 28.3 | 71.5 | NR | |
No | 71.7 | 28.5 | NR |
De Bree and Helm’s review [13] does not describe or summarize these types of data, even if they are reported more or less analytically in the original individual studies. Anatomopathological characteristics and FIGO stage seemed rather similar between studies reporting these specific data: the absolute majority of patients had high-grade (G3) and FIGO stage III/IV tumors. Except for Deraco et al.’s study [35], which includes only cases treated as front line, all other studies refer to various settings in which PRT plus HIPEC was performed: distribution of setting results was sufficiently homogeneous.
Chemosensitivity to platinum was analyzed in different ways in four studies: HYPER-O evaluated the response to chemotherapeutic treatments performed as front line in all settings. Bakrin [29] described the pre-HIPEC response exclusively in patients with recurrent or persistent carcinomatosis. In our patients, chemosensitivity was evaluated for adjuvant CHT post-HIPEC performed after both primary and secondary cytoreduction; in addition, NACT sensitivity was considered in the primary setting. This evaluation was also performed in the MIIS.
PCI score differs significantly between studies: in ours and in that of Deraco et al., the median PCI score was the highest; the French study [28] reported the lowest. Optimal cytoreduction scores were homogeneous among these studies. In all studies except ours, combinations of drugs were used for HIPEC, with platinum-based drugs being the most commonly used. Drug doses were very variable: CDDP doses ranged from 50 to 100 mg/m2. HIPEC duration varied from 30 to 120 min according to the drug used. Both open and closed HIPEC techniques were used, except for in Deraco et al.’s and our studies, in which only the closed technique was applied. In all studies, protocols provided for adjuvant treatments, even if the percentage of their application was significantly different.
20.4.1 Survival and Prognostic Factors
The majority of studies analyzed survival in relation to various settings in which CSR plus HIPEC was performed. Deraco et al.’s study is an exception, as it is totally dedicated to front-line cases. Our monocentric study, the MIIS (unpublished), and de Bree and Helm’s [33] review describe similar long-term results after primary and secondary CRS respectively (Tables 20.4 and 20.5). For primary CRS, survival at 5 years was remarkably variable in the de Bree and Helm review, ranging from 33% to 84%; in multicentric analyses and in the monocentric study, survival was more homogeneous, with a median value almost 50 % at 5 years for primary CRS and 40 % for secondary CRS. The HYPER-O registry [34] demonstrated significantly lower survival values in secondary CRS (18 % at 5 years and a median of 23.5 months) compared to all other studies. Across studies, PFS values at 5 years range from 13 % to 50 %; in the monocentric study, PFS is higher than other studies and closer to OS (Fig. 20.5).
Tab 20.4 Part I
PRT plus HIPEC in ovarian carcinomatosis treatment. Survival and prognosis factors
Multicentre Studies | ||||
|---|---|---|---|---|
Study [Reference] | HYPER-O [34] | |||
5 yr OS | 5 yr PFS | median OS | median PFS | |
SETTING | % | % | months | months |
frontline | 33,3 | 19,7 | 41,7 | 24,8 |
interval debulking | 50,2* | 9,6* | 68,6* | 16,8* |
consolidation | 42,4* | 24,2* | 53,7* | 29,6* |
recurrence | 18 | 9,6 | 23,5 | 13,7 |
Primary | 25,4 | 13 | 30,3 | 13,7 |
Recurrence | – | – | – | – |
Maximal cytoreduction | ||||
CC 0 (primary) | 26,7 | – | 37 | – |
CC 0 (recurrence) | – | – | – | – |
Prognostic factors | Univariate or Multivariate Analyses – p value | |||
CC score | 0.025 | |||
PCI | nr | |||
PS | nr | |||
Platinum response | 0.048 | |||
setting | ns | |||
blodd loss | 0,005 | |||
ca 125 | nr | |||
Lymph-node metastases | nr | |||
age | nr | |||
HIPEC drugs number | nr | |||
HIPEC drug type(carboplatin) | 0.011 | |||
duration of perfusion (90 min) | 0,047 | |||
Study [Reference] | DERACO [35] | |||
5 yr OS | 5 yr PFS | median OS | median PFS | |
SETTING | % | % | months | months |
frontline | 60,7 | 15,2 | not reached | 30 |
Fig 20.4 Part II
PRT plus HIPEC in ovarian carcinomatosis treatment. Survival and prognosis factors
Multicentre Studies | ||||
|---|---|---|---|---|
Study [Reference] | BAKRIN [29]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||