•
T stage of primary (T4)
•
Lymph-node status (N1-2)
•
Mucinous/signet-ring-cell histology
•
Right-sided tumors
•
Positive peritoneal cytology
•
Emergency/nonradical surgery
•
Perforated/ruptured primary
•
Ovarian metastases
•
Previously resected peritoneal nodules
•
Young age
19.2 Role of Systemic Chemotherapy
Systemic chemotherapy has been considered for many years the only therapeutic option for CRC patients with PC. In the era of 5-fluorouracil (5-FU)-based therapy, the results of systemic chemotherapy for CRC PC were discouraging [4, 6]. Considering a multivariate analysis on predictors of outcome in patients with CRC stage IV disease treated with 5-FU, PC is associated with the worst outcome (median survival 7.7 months) with respect to patients (median survival 11.6 months) with other site of metastases (liver, lung, lymph nodes) [13]. Significant progress has been made in the medical management of metastatic CRC. The introduction of oxaliplatin and irinotecan to 5-FU-based regimens, coupled more recently with targeted biological therapy (bevacizumab and cetuximab) has improved outcomes for PC patients, leading to a median survival > 20 months [14–19]. The more adverse outcome of PC with respect to other sites of metastatic disease was also confirmed within modern chemotherapy combination treatments, with an estimated 30 % reduction in overall survival (OS) for patients with PC with respect to other unresectable metastatic sites [15]. A possible explanation can be that only patients with more advanced disease were included in those trials, as “no measurable disease” by imaging techniques is a frequent ineligibility criterion for randomized controlled trials (RCT). A part from these considerations, median survival of patients with PC without other sites of metastasis (isolated PC) ranges from 21.8 to 23.9 months [14, 18], suggesting that metastatic CRC confined in the abdominal cavity represents a distinct biological entity with a more favorable prognosis. Although these retrospective and uncontrolled data indicate that modern systemic chemotherapy has led to a better outcome for patients with PC, prospective studies investigating its effect on the subset of patients with isolated PC are still lacking, as are data on the most efficient treatment combination for this clinical entity.
19.3 Role of Surgery
Results of surgical resection for stage IV CRC in recent decades has led to the change in indications for radical surgery of metastases, which are no longer being considered an absolute contraindication. In selected patients with colorectal liver metastasis, though in the absence of prospective randomized clinical trials, radical resection is now considered the standard of care, as a survival benefit has been clearly deduced from historical controls since the late 1990s [20]. For extrahepatic metastatic disease (lung, lymph node, peritoneum), a reasonable number of published studies suggest a potential survival benefit in a highly selected group of patients [21, 22]. The use of surgery for colorectal PC was once restricted to complication palliation, such as intestinal obstruction or perforation. A potential beneficial role of radical surgery in a subset of CRC patients with peritoneal implants has been suggested by some retrospective studies [23–26]. Radical removal of limited peritoneal implants (around the primary tumor or on the ovarian surface) is associated with improved prognosis, whereas gross residual disease and tumor load at the completion of surgery are factors negatively affecting survival.
19.4 Role of Surgery plus Intraperitoneal Chemotherapy
Based on the rationale of the “metastatic insufficiency” of some forms of PC from CRC, the combined approach based on maximal CRS and IP-administered chemotherapy (IP-CHT) was proposed by Sugarbaker, who first demonstrated the feasibility of this treatment and identified prognostic factors for patient selection [27]. After this pioneering experience, several studies (single institution and multicenter) have investigated the utility of CRS associated with IP drug delivery for selected patients with CRC peritoneal involvement, in most cases under hyperthermic conditions [hyperthermic intraperitoneal chemotherapy (HIPEC)] [28, 29]. These studies reported longer survival (median range between 12.8 and 60.1 months) for patients undergoing surgery plus HIPEC versus historical controls treated with systemic chemotherapy alone. However, these results should be interpreted with caution. The selection criteria were extremely heterogeneous; in some trials, patients with hematogenous (liver) metastases were also included in the final analysis, whereas in others, more favorable histology (appendix, pseudomyxoma) was present. Moreover, the timing of IP-CHT (intra-/postoperative), HIPEC method (closed vs. open), and drug type and dosage varied widely among studies. Nonetheless, results of these studies appear much better than those reported in historical control patients treated with chemotherapy alone. These encouraging results were confirmed by a randomized controlled trial promoted by The Netherlands Cancer Institute, which compared standard [5-FU/leucovorin (LV) with or without palliative surgery] with experimental (CRS plus HIPEC) treatment [30]. This trial showed a significant advantage in survival (12.6 vs. 22.3 months) in favor of CRS plus HIPEC group and was stopped prematurely for ethical reasons. These results were confirmed by other comparative studies [17–19, 31] (Table 19.2). Level of evidence at present justifies the use of CRS plus HIPEC in selected patients in centers with significant experience in treating peritoneal surface malignancies, preferably in the context of prospective trials. The decision-making process should be based upon defined selection criteria, which should be discussed in a multidisciplinary meeting involving surgeons, oncologists, and radiologists. Different scoring systems have been proposed to identify with good accuracy patients with severe colorectal PC and predicted short survival outcome. However, none of studies was prospectively tested [32, 33]. A full explanation of potential risks and benefits of CRS plus HIPEC should be given to the patients, explaining treatment alternatives and taking into account the individual motivation in undergoing this treatment (Fig. 19.1).
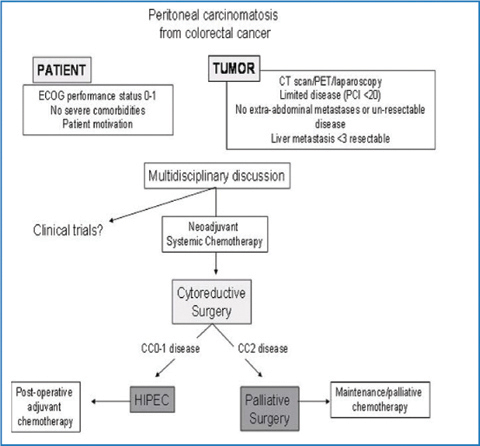

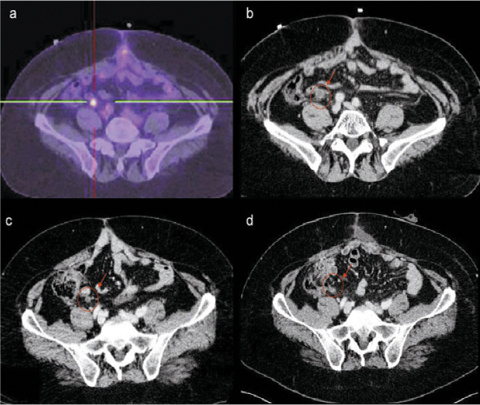
Fig. 19.1
Algorithm for selecting and treating patients with colorectal peritoneal carcinomatosis at Veneto Institute of Oncology. HIPEC, hyperthermic intraperitoneal chemotherapy; ECOG, Eastern Cooperative Oncology Group; CT, computed tomography; PET, positron emission tomography; PCI, Peritoneal Cancer Index; CC, Completeness of Cytoreduction score
Table 19.2
Results of modern chemotherapy in colorectal peritoneal carcinomatosis
Study [Reference] | Study type | Study period | Patients (N) | Chemotherapy type | Extraperitoneal metastasis | Median overall survival (months) | Median progression-free survival (months) |
|---|---|---|---|---|---|---|---|
Elias et al. [18] | Retrospective analysis of institutional multihospital cancer registries | 1998–2003 | 48 | Oxaliplatin-irinotecan based ± bevacizumab/cetuximab | No | 23.9 | NA |
Franko et al. [19] | Retrospective analysis of institutional multihospital cancer registries | 2001–2007 | 38 | Oxaliplatin-irinotecan based ± bevacizumab/cetuximab | Yes | 16.8 | NA |
Klaver et al. [14] | Eindhoven cancer registry analysis | 2006–2009 | 136 | Not reported (national guidelines) | No | 21.8 | 15 |
Franko et al. [15] | Retrospective analysis of two phase III trials of North Central Treatment Group (N9741-N9841) | 1998–2003 | 364 | Oxaliplatin-irinotecan based | Yes | 15.7 | 5.8 |
Klaver et al. [16] | Retrospective analysis of two phase III trials of the Dutch Colorectal Cancer Group (CAIRO 1 and 2) | 2003–2005 | 81 | Oxaliplatin-irinotecan based ± | Yes | 15.2 | 7.2 |
Chua et al. [17] | Retrospective analysis of multicentre prospective collected databases | 1988–2009 | 32 | Oxaliplatin-irinotecan based ± bevacizumab/cetuximab | No | 23 | NA |
19.5 Selection Criteria
19.5.1 Cytoreduction Completeness
The maximum size of residual disease remaining after surgical cytoreduction has a strong prognostic value for colorectal PC [34]. Median survival of patients with incomplete surgery (i.e., residual disease > 2.5 mm) and IP-CHT is only 8 months. This observation leads to the conclusion that it is necessary to achieve complete cytoreduction before treating a patient with HIPEC, as confirmed by a consensus statement built on expert opinion [35]. The possibility of obtaining complete cytoreduction depends on established clinical and radiological criteria valid for all types of peritoneal malignancies selected for HIPEC. Biliary tract, ureteral, and multiple small-bowel obstruction and extensive small-bowel mesentery and gastrohepatic ligament involvement represent situations in which direct penetration beyond the peritoneal barrier into unresectable or marginally resectable structures make a complete or near complete cytoreduction unlikely and are associated with an unacceptable risk of complication [36].
A learning-curve effect on cytoreduction has been demonstrated, with the zenith of the learning curve (graded by the percentage of complete cytoreductions) reached after approximately 130 procedures. [37]. On this basis, the complete cytoreduction rate reflects a center’s experience on patient selection and treatment expertise, meaning that all colon cancers with peritoneal dissemination should be referred to an experienced peritoneal-surface malignancy center for accurate clinical, radiological, and—possibly—surgical assessment of carcinomatosis resectability.
19.5.2 Peritoneal Diffusion
Disease extent, calculated as the number of abdominal regions involved and volume of nodules in each region, represents an important prognostic criteria in colorectal PC patients selected for CRS plus HIPEC. Among the different scoring systems, the Peritoneal Cancer Index (PCI) is the most frequently used for staging and was elected the best intraoperative staging system in treating peritoneal surface malignancies [38], although other staging systems are proven to have a prognostic value for colorectal PC [39, 40]. The prognostic cutoff values for PCI were investigated in a large multi-institutional retrospective study of 523 patients treated with CRS and IP-CHT between 1990 and 2007. When PCI is > 20, median survival is 18 months, which is no longer than after systemic chemotherapy alone [34]. For these reasons, PCI > 20 is now considered a relative contraindication to CRS plus HIPEC, a statement confirmed by one international expert consensus [36]. Although computed tomography (CT) and positron emission tomography (PET) technology and expertise in PC could improve in the near future, at present, scores of preoperative radiological PCI formulation before surgical exploration significantly underestimate intraoperative PCI [41]. It has been calculated that 12 % of patients selected for CRS plus HIPEC after CT scan became ineligible for treatment after surgical exploration [42]. Laparoscopic exploration has been proposed as a complementary method for predicting PCI in patients selected for CRS plus HIPEC [43]. Because a clear underestimation of the laparoscopic PCI score compared with open PCI has been shown [44, 45], a more defined clinical and radiological selection process is necessary to understand which patients are eligible for pre-CRS plus HIPEC laparoscopy workup. This would avoid unnecessary laparotomy in a higher percentage of cases and contribute to a more cost-effective treatment and better QoL for these patients.
19.5.3 Primary tumor
Primary tumor site is considered an important prognostic factor. Patients with rectal cancer treated with CRS and peritoneal IP-CHT have a worse survival rate with respect to colon cancer [46, 47]. Moreover, aggressiveness of primary tumor histology (signet-ring-cell histology, grading, lymph node involvement) suggests a worse prognosis [34, 48–50].
19.5.4 Extraperitoneal Disease
CRS plus HIPEC is usually contraindicated in the presence of systemic disease, and colorectal patients with peritoneal carcinomatosis associated with extraperitoneal metastases are usually deemed not suitable for treatment and are referred to an oncologist for systemic chemotherapy. Surgery for limited and stable metastatic disease has sometimes been offered to patients with multiple disease sites (liver, lung, peritoneum), but the issue remains under investigation, and results of the few available studies are influenced by a high selection bias [21]. A single-center experience reported a 28 % 5-year survival rate in patients who underwent an R0 resection of extrahepatic disease simultaneously with hepatectomy for colorectal liver metastasis [51]. Although the reported experience remains limited, an increasing number of studies on this subject have been published [52]. Patients with metastases in the liver and peritoneum who were selected for curative resection and HIPEC showed a trend toward a lower OS when compared with patients with isolated peritoneal disease treated with CRS plus HIPEC. However, patients with liver and peritoneal metastases show a better survival trend after CRS plus HIPEC when compared with modern systemic chemotherapy alone. Hence, there is insufficient evidence to exclude patients with liver and peritoneal disease from a potentially curative treatment. In these patients, the selection process should be more accurate, taking into account the potential added morbidity of liver resection and that only patients with low peritoneal burden (PCI < 10) and liver disease (< 3 metastases) will probably reap benefits from this combined approach [53–55].
19.6 Italian Experience
The Italian experience is collected in a prospective database that includes operative, postoperative, and follow-up data of patients treated in five Italian centers and represents the largest Italian study on multimodal treatment of colorectal PC. A recent update investigating 146 consecutive patients treated during from 1995 to 2007 confirms that the cytoreduction completeness represents the most important prognostic factor, along with the presence of liver metastases resected at any time during the disease, of unfavorable sites such as small intestine, hepatic hilum, diaphragm, and of gross involvement of retroperitoneal lymph nodes [56].
19.7 Open Questions
19.7.1 Need for Treatment Standardization
Among the oncological community, a main criticism regarding benefits of IP administration of drugs for colorectal carcinomatosis is the absolute lack of standardization. There is a high variability in HIPEC modality (open vs. closed), chosen drugs (mitomycin vs. oxaliplatin based), drug dosage, perfusate volume, inflow temperature, and perfusion duration [57]. Both mitomycin C and oxaliplatin are considered for IP administration for their high molecular weight, resulting in a high IP concentration and limited systemic absorption and toxicity. For mitomycin C, a dose-escalation study fixed the maximum tolerated dose as single agent at 35 mg/m2 [58]. For oxaliplatin, a dosage of 460 mg/m2 in 2 l/m2 of 5 % dextrose at 42–44 °C over 30 min was recommended [59]. In that protocol, IV administration of 5-FU (400 mg/m2) and LV (20 mg/m2) 1 h before HIPEC was proposed to enhance IP-administered oxaliplatin activity. Combination drug therapies with a mitomycin C and oxaliplatin dose reduction have been studied, some of which have been adopted by centers worldwide, although not always tested in a phase I trial. The combination of mitomycin C (15–20 mg/m2) and doxorubicin (15 mg/m2) has been tested in the USA and Germany [60]. In Italy, the preferred drug combination is mitomycin C (3.3 mg/m2/l) plus cisplatinum (25 mg/m2/l) for 60–90 min [56]. In a few uncontrolled studies comparing mitomycin C and oxaliplatin-based regimens, no clear difference in toxicity (hematological, renal), postoperative complication rate, disease free and OS were detected when comparing the two HIPEC protocols [60, 61].
In absence of level I evidence, the choice of drugs and technique for HIPEC administration remains mainly based on the tradition of the center rather than on a critical evaluation of toxicity and efficacy. While waiting designed prospective trials to determine the best HIPEC protocol, the first attempt for achieving a consensus on this issue emerged in the fifth international workshop on peritoneal surface malignancies held in Milan in 2006 [62]. In this context, substantial agreement was obtained and published by the American Society of Peritoneal Surface Malignancies (ASPSM) regarding the optimal HIPEC delivery method for colorectal carcinomatosis [63]. The standardization of HIPEC delivery protocols in colorectal PC through consensus documents and multiinstitutional registries is the first step toward a clear definition of the optimal treatment regimen in relation to morbidity, mortality, and long-term outcome.
19.7.2 Comparing CRS plus HIPEC and New Systemic CHT
Despite the substantial benefits of CRS plus HIPEC with respect to systemic chemotherapy in colorectal PC demonstrated in a RCT and in several uncontrolled comparative studies, significant scepticism remains with regard to the wide applicability of CRS plus HIPEC in terms of safety and efficacy. Improved response and prolonged OS rates of patients with stage IV CRC obtained after the introduction of modern chemotherapy (median survival > 20 months) raises the question of whether CRS plus HIPEC remains the optimal treatment option for colorectal carcinomatosis. The question appears even more urgent after the introduction of biologic therapy [vascular endothelial growth factor (VEGF) and epithelial growth factor receptor (EGFR) inhibitors], is reported to improve survival outcome compared with standard treatment alone [17, 64]. The role of modern systemic chemotherapy in colorectal PC is poorly investigated, and few data are available regarding patients with isolated peritoneal involvement. A prospective study on systemic chemotherapy restricted to peritoneal carcinomatosis has not yet been done. The question of whether CRS plus HIPEC offers a significant advantage in terms of survival compared with a highly selected and well-matched group of patients with colorectal PC treated with “modern” chemotherapy regimens alone remains unanswered, and some trials attempting to clarify this issue are ongoing at the time of this writing (Table 19.3). CRS plus HIPEC should be matched in the near future with the improved results of systemic chemotherapy, keeping in mind that treatmentrelated mortality and morbidity, side effects, and QoL play a pivotal role in therapeutic planning in a population with a relatively short life expectancy.
Table 19.3
Registered trials on CRS plus HIPEC for peritoneal carcinomatosis from colorectal cancer
Title | ID number | Sponsor | Country | Phase | Primary end point | Study start date | Status |
|---|---|---|---|---|---|---|---|
A randomized phase-III study comparing cytoreductive surgery plus intraperitoneal chemotherapy versus modern systemic chemotherapy in colorectal peritoneal carcinomatosis | ClinicalTrials.gov ID: | Uppsala University | Sweden | III | Overall survival | June 2003 | Completed |
Cytoreductive surgery associated with hyperthermic chemotherapy: intraperitoneal versus systemic chemotherapy in the treatment of resectable colorectal carcinomatosis. IOV-CAR-CRC-1-2012 | EudraCT no: 2012-004058-27 | Veneto Oncology Institute, Padua | Italy | II | Overall survival | January 2013 | Recruiting |
Multicentric phase III trial comparing simple follow-up to exploratory laparotomy plus “in principle” HIPEC in colorectal patients initially treated with surgery and adjuvant chemotherapy who have a high risk of developing colorectal peritoneal carcinomatosis. ProphyloCHIP | ClinicalTrials.gov ID: NCT01226394 EudraCT no.: 2009-015598-11 | Gustave Roussy, Cancer Campus, Grand Paris | France | III | Disease-free survival | April 2010 | Recruiting |
Randomized phase II study to evaluate an audit approach: surgical systematically associated with intraperitoneal chemohyperthermia and possible cytoreductive surgery versus standard follow-up in patients at high risk of developing peritoneal carcinomatosis from colorectal carcinoma | EudractCT no. 2012- 002739-27 | University of Milan, “Luigi Sacco” Hospital | Italy | II | Overall survival | June 2012 | Recruiting |
Prospective randomized trial evaluating mandatory second look surgery with HIPEC and CRS versus standard of care in subjects at high risk of developing colorectal peritoneal metastases | ClinicalTrials.gov ID: NCT01095523 | National Cancer Institute (NCI) | USA | II | Overall survival | January 2010 | Withdrawn |
Multimodality treatment including pre- and postoperative systemic chemotherapy plus cetuximab, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from wild type K-ras colon cancer: a prospective multicenter phase II Study. COMBACT trial | ClinicalTrials.govID: NCT01540344 EudraCT no.: 2009 -014040-11 | University of Regensburg | Germany | II | Progression-free survival | October 2010 | Recruiting |
Neoadjuvant chemotherapy, cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin (NACHO trial) | EudraCT no.: 2010- 020787-37 | University Medical Center, Groningen | Netherlands | II | Safety of neo-adjuvant chemotherapy | November 2011 | Recruiting |
Phase II study comparing normothermic versus hyperthermic intraoperative chemoperfusion with oxaliplatin in patients with peritoneal metastases from appendiceal or colon cancer | ClinicalTrials.govID: NCT01575730 | University Hospital, Ghent | Belgium | II | Morbidity/mortality, oxaliplatin pharmacokinetics | June 2012 | Recruiting |
Phase III study evaluating the use of systemic chemotherapy and chemohyperthermia intraperitoneal preoperatively (CHIP) and after maximum resection of peritoneal carcinomatosis originating with colorectal cance (Prodige 7/ACCORD 15) | ClinicalTrials.gov ID: NCT00769405 EudraCT no: 2006-006175-20 | UNICANCER | France/Spain | III | Overall survival | February 2008 | Recruiting |
Randomized phase 2 study comparing second-look laparoscopy to standard follow-up in patients with no radiologic evidence of disease at 6 months after complete resection of colorectal mucinous carcinoma | ClinicalTrials.gov ID: NCT01628211 | National Cancer Institute, Naples | Italy | II | Overall survival | April 2012 | Recruiting |
19.7.3 Role of Adjuvant Chemotherapy
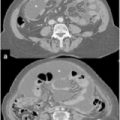
Systemic adjuvant chemotherapy in a patient population with carcinomatosis of colonic origin selected for CRS and IP-CHT showed a positive prognostic impact in two large registry studies [34, 48]. Half of the patients with isolated PC treated with CRS plus HIPEC showed systemic recurrence during their life course, and the rationale for treating those patients with systemic chemotherapy was mainly directed at preventing hematogenous metastases (liver, lung, bone). Moreover, neoadjuvant chemotherapy allows for better patient selection for CRS plus HIPEC, excluding those with systemic progression and considering only those with a more favorable tumor biology (better drug responsiveness, lack of metastatic potential). The activity of systemic chemotherapy on colorectal peritoneal nodules is largely unknown. As for rectal cancer and liver metastasis, the radiological and histological response of peritoneal nodules to neoadjuvant chemotherapy can theoretically provide important information on tumor chemosensitivity and suggest the optimal postoperative regimen after surgery (Figs. 19.2 and 19.3). At present, the few available data show that failure to respond to systemic adjuvant treatment before CRS plus HIPEC is not necessarily associated with an unfavorable outcome [65, 66]. For this reason, failure to respond to previous adjuvant systemic treatment should not be considered an exclusion criterion for treatment with CRS plus HIPEC. A possible explanation for the supposed little efficacy of systemically administered drugs could be the poor vascularization of peritoneal nodules, which prevents a therapeutic drug concentration. A pilot study aimed at evaluating the response rate and characteristics of patients with initially unresectable colorectal PC seems to confirm a substantial unresponsiveness to systemically administered chemotherapy [67]. In that study, all patients were evaluated with laparoscopy before and after chemotherapy; and in none of them was systemic administration of chemotherapy able to convert unresectable PC into resectable PC, a condition that would have made those patients potentially suitable candidates for CRS plus HIPEC. At laparoscopy, 78 % of patients showed progressive disease, and no macroscopic response was documented. Interestingly, laparoscopic evaluation showed an important discrepancy with respect to that obtained with radiological evaluation. These findings suggest a substantial unreliability of disease response assessment based on preoperative imaging alone.