Cholangiocarcinoma is a diverse group of rare but highly fatal malignancies arising from the biliary tract epithelium. According to their anatomical origin in the biliary tract, cholangiocarcinomas can be subdivided into three distinct categories, namely intrahepatic, perihilar, and distal cholangiocarcinoma. Besides definitional purposes, considering each category of different biologic characteristics and clinical course, such a classification is useful in diagnostic approach and clinical management.
In this chapter, epidemiology, clinical presentation, available diagnostic modalities, and finally staging and clinical management of perihilar cholangiocarcinoma are discussed. Intrahepatic cholangiocarcinoma and distal cholangiocarcinoma are discussed separately.
Cholangiocarcinoma was first reported by Durand-Fardel in 1840 and originally referred only to primary tumors of the intrahepatic bile ducts.1–3 More recently, the term “cholangiocarcinoma” has been used to refer to all primary tumors arising from the malignant proliferation of the epithelial cells lining the biliary tract, including both intrahepatic and extrahepatic ducts.4
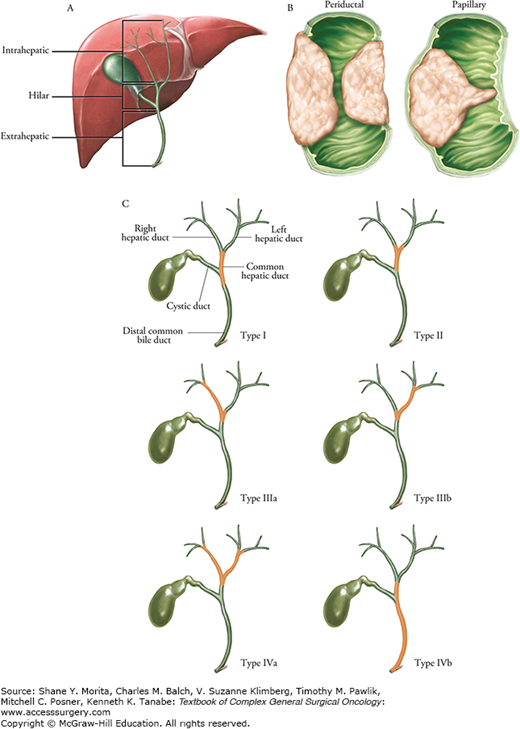
Cholangiocarcinomas are classified according to their anatomical origin as intrahepatic (located proximally to the second-degree branches of left and right bile ducts within the liver), perihilar (confined to the area between the second-degree bile ducts and the insertion of cystic duct into the common bile duct), or distal cholangiocarcinoma (localized within the pancreatic portion of the common bile duct, distal to the insertion of the cystic duct).4,5 Perihilar cholangiocarcinoma, first described by Klatskin in 1965,6 can be further subdivided into four types (Bismuth–Corlette classification) based on the distribution of the bile duct involvement7,8 (Fig. 133-1). The term “Klatskin tumor” or hilar cholangiocarcinoma (HC) typically refers to tumor involving the common hepatic duct bifurcation.
FIGURE 133-1
Classification of cholangiocarcinoma. A. The classification of cholangiocarcinoma can be based on anatomic location: intrahepatic, hilar, or extrahepatic. B. Lesions can be further characterized as mass-like, periductal, or intraductal. C. Bismuth classification for hilar lesions.8 Type I, involvement of hepatic duct below the bifurcation; Type II, involvement of bifurcation without invasion of second-order ducts; Type IIIa, involvement of right-sided ducts into second order, but with sparing of left second-order ducts; Type IIIb, involvement of left-sided ducts into second order, but with sparing of right second-order ducts; Type IV, involvement of bilateral second-order ducts (IVa) and/or skip lesions (IVb).
Most cholangiocarcinomas are adenocarcinomas (>90%), with rare occurrence of other histologic subtypes (i.e., squamous cell carcinoma, signet-ring carcinoma, papillary adenocarcinoma, etc.), and can be graded as well-, moderately, or poorly differentiated tumors (more commonly well- and moderately differentiated).9–12 Macroscopically, the three distinct subtypes of cholangiocarcinoma include sclerosing, nodular, and papillary.13,14 The majority of perihilar cholangiocarcinomas are sclerosing tumors that are characterized by annular thickening of the bile duct, extensive fibrosis of periductal tissues, and a dense desmoplastic reaction.12–14 These characteristics make preoperative diagnosis of perihilar cholangiocarcinomas by tissue sampling and cytology difficult and contribute to the technical difficulties encountered during surgical resection of these tumors. A tumor of the nodular subtype presents as a firm irregular nodule that projects into the bile duct lumen.12,14 Many times the tumor has characteristics of both subtypes and is therefore classified as nodular-sclerosing tumor. The papillary subtype is uncommon and usually presents as a mobile intraductal soft and friable tumor with a well-defined stalk. It presents relatively earlier than other subtypes and is less likely to invade the bile duct wall. As a result, the papillary tumors have higher rate of resectability and more favorable prognosis than nodular and sclerosing subtypes.
The Liver Cancer Study Group of Japan has proposed an alternative classification of cholangiocarcinoma, which, in addition to histology, incorporates growth characteristics that have prognostic implication in these tumors.10 In this model (applied to both intrahepatic and extrahepatic masses), tumors are classified into mass-forming, periductal-infiltrating, and intraductal-growing types. The majority of perihilar cholangiocarcinomas are categorized in periductal infiltrating type, where the tumor infiltrates along the main and intrahepatic bile ducts.15 Intraductal papillary neoplasms are often well-differentiated with early onset of symptoms (most commonly obstructive jaundice) resulting in earlier diagnosis and a more favorable prognosis.
Globally, cholangiocarcinoma accounts for approximately 3% of all gastrointestinal malignancies15,16 with an annual incidence rate of 1 to 2 cases per 100,000 population in the United States.16–18 Cholangiocarcinoma is the most common primary biliary tract malignancy and second most common primary hepatic tumor (after hepatocellular carcinoma).15,16,19 The most common type of cholangiocarcinoma is perihilar cholangiocarcinoma (50% to 60%), followed by distal and intrahepatic types constituting 30% to 40% and 5% to 10% of all cholangiocarcinomas, respectively.20,21
Several comprehensive epidemiological studies have shown that the incidence and mortality rates of extrahepatic cholangiocarcinomas are declining worldwide, while those of intrahepatic cholangiocarcinoma are rising (although this pattern is not universal). According to the Surveillance Epidemiology, and End Results (SEER) data, the age-standardized incidence rate of extrahepatic tumors in the United States decreased from 1.08 per 100,000 in 1979 to 0.82 per 100,000 in 1998. A similar fall was observed in age-standardized mortality rate of the tumor in the same period of time (from 0.6 to 0.3 per 10,000).26 However, these data should be interpreted with caution as they may be confounded by the inclusion of other biliary cancers in the analysis (specifically, gall bladder cancer, where the increase in incidence may in part be accounted for by increased rate of cholecystectomy).19,26
Most patients with cholangiocarcinoma are in their seventh decade of life at the time of diagnosis17,27 and is slightly more prevalent in males.16,26 In general, there is a direct correlation between the age of the patient and the incidence of cholangiocarcinoma.15 However, in patients with underlying primary sclerosing cholangitis (PSC) or congenital biliary tract disease (e.g., choledochal cysts), cholangiocarcinoma tends to present earlier, around 30 to 50 years of age.
Although most cases of cholangiocarcinoma occur sporadically, there are several well-defined risk factors that are associated with an increased incidence of cholangiocarcinoma.3,27,31 Geographic disparity with respect to incidence rate of cholangiocarcinoma is related to risk factor variation. In Western countries, PSC is the most common predisposing condition, especially for perihilar cholangiocarcinoma.2,27 Approximately 30% of cholangiocarcinomas are diagnosed in patients with PSC. The annual incidence of cholangiocarcinoma in patients with PSC is between 0.6% and 1.5 % per year, with a lifetime risk of 5% to 15%.32–36 This rate has been reported to be as high as 40% in explanted liver specimens.37 Although a correlation was initially presumed between the duration of the underlying inflammatory disease (PSC) and the incidence of cholangiocarcinoma, about one-third of patients with PSC develop cholangiocarcinoma within 2 years of diagnosis, thereby refuting this association.28,33,37,38 In patients with PSC, approximately two-thirds will also have inflammatory bowel disease, most commonly ulcerative colitis.38 There is no data supporting an association between the risk of developing cholangiocarcinoma and the severity, duration, or extent of inflammatory bowel disease.29,38 Moreover, treatment of underlying colitis does not alter the incidence of cholangiocarcinoma in these patients.
Congenital malformations of the biliary tree (e.g., choledochal cysts, congenital hepatic fibrosis, and Caroli’s syndrome) are another well-known risk factor for developing cholangiocarcinoma, with approximately 15% to 20% risk of malignant change after the second decade of life.2,39,40 In some studies, the incidence of cholangiocarcinoma in patients with untreated cysts is reported to be as high as 28%.39,40 Although the exact mechanism of carcinogenesis is unclear, it has been suggested that this is related to chronic inflammation of the biliary tree caused by pancreatic enzyme reflux, biliary stasis, and activation of bile acids caused by the pancreaticobiliary junction malformations commonly seen in these patients.2,27,41
Bile duct adenomas and multiple biliary papillomatosis (characterized by multiple intrahepatic bile duct adenomatous polyps) are other biliary tract diseases shown to be associated with the development of cholangiocarcinoma.42,43
Chronic biliary tract inflammation caused by hepatolithiasis (intrahepatic biliary duct stones, also called “recurrent pyogenic cholangitis”)44,45 and liver parasitic infections (such as Opisthorchis viverrini and Clonorchis sinensis)46–49 are relatively common risk factors for cholangiocarcinoma (mainly intrahepatic type) in some Asian countries (especially Taiwan and Thailand); however, their occurrence in Western countries is rare.
Hepatitis B and C are established risk factors for hepatocellular carcinoma. The association between hepatitis B and C viruses and cholangiocarcinoma has been shown by different studies from Korea, Italy, and Japan.50–52 In the United States, at least one case-controlled study and one large cohort identified hepatitis C as potential risk factor for developing intrahepatic cholangiocarcinoma.17,30,53,54 The contribution of hepatitis C and hepatitis B in development of cholangiocarcinoma varies geographically. While hepatitis C is more prevalent in Western countries, hepatitis B is more common in Asian countries. A recent meta-analysis by Palmer and Patel,55 showed that cirrhosis, hepatitis B, and hepatitis C are major risk factors for development of intrahepatic cholangiocarcinoma with odds ratios of 22.9, 5.1, and 4.8, respectively. Of note, cirrhosis regardless of its etiology also has been associated with developing intrahepatic cholangiocarcinoma.17
There is increasing evidence to suggest an association between cholangiocarcinoma and obesity, diabetes and alcohol use, and risk factors implicated in many other cancers.27,29,55 In addition, increased incidence following exposure to chemical carcinogens such as Thorotrast, dioxins, and nitrosamines has been demonstrated in some studies.
Several studies have shown an association between cholangiocarcinoma and a number of mutations involving both oncogenes (K-ras, c-myc, epidermal growth factor receptor (EGFR), beta-catenin (CTNNB1)) and tumor suppressor genes (p53, SMAD4, APC, CDKN2A).3,4,59–61 Overexpression of some proto-oncogenes (c-met and c-erbB-2) has been linked to metastatic transformation of intrahepatic tumors and a more aggressive tumor phenotype.62–64 While these findings are promising for the creation of gene-targeted therapies, the molecular changes associated with the pathogenesis of cholangiocarcinoma are significantly less well characterized than that of other gastrointestinal malignancies such as colorectal cancer. A detailed review of the molecular pathogenesis of cholangiocarcinoma is beyond the scope of this chapter and interested readers are referred to selected review articles.4,14,63,65 In summary, the development of cholangiocarcinoma, similar to many other malignancies, is a stepwise process resulting from the interaction between environmental factors and host genetic factors.2
The clinical features of the perihilar cholangiocarcinoma depend on the stage and location of the tumor.20,66 In the majority of patients (>90%), the disease remains clinically silent until it presents at advanced stages with obstructive jaundice (secondary to obstruction of the biliary drainage system by the tumor).9,66,67 Symptoms of progressive cholestasis, such as pruritus, dark urine, and clay-colored stools, typically accompany the appearance of the jaundice.66,67 However, in patients with incomplete biliary obstruction of the right or left hepatic duct or segmental duct, jaundice might not be the presenting feature. This delays the diagnosis of the tumor and may lead to the development of a hypertrophy–atrophy complex, presenting as the palpable prominence of one hepatic lobe.67,68 The main reason for this finding is unilobar biliary obstruction resulting in unilateral hepatic lobe hypertrophy with contralateral hepatic lobar atrophy and is often accompanied by ipsilateral vascular encasement.69 In the case of intraductal papillary tumors, the patient might describe history of intermittent jaundice (resulting from sloughing off small fragments of a friable tumor or a ball-valve effect of a mobile tumor). Systemic symptoms of malignancy such as fatigue, malaise, weight loss, fever, and anorexia are observed in up to one-third of the patients at the time of presentation.66,67
Rarely, the evaluation of vague abdominal pain or abnormal liver tests leads to the diagnosis of cholangiocarcinoma in early stages of the disease. Abdominal pain related to the tumor is generally described as a constant dull ache located in the right upper quadrant without radiation. Cholangitis is an unusual presentation (in up to 10% of patients) in the absence of previous biliary instrumentation.9,66
The diagnosis of cholangiocarcinoma in patients with underlying PSC can be especially challenging. Development of cholangiocarcinoma in these patients tends to present with rapid deterioration of clinical status, fall in performance status, and increasing cholestasis.70
Physical exam findings in most patients include jaundice and hepatomegaly.20 Less frequently, a distended gall bladder may be palpable in a jaundiced patient (Courvoisier’s sign).71 However, this finding is more common in distal cholangiocarcinomas, and in perihilar type the gall bladder is usually decompressed and nonpalpable.71 Signs of portal hypertension may be present in the case of long-standing biliary obstruction, portal vein involvement, or both.
It should be emphasized that none of the clinical findings reviewed in the previous section is specific for the diagnosis of the cholangiocarcinoma. Features of cholestasis can be seen in a wide spectrum of benign and malignant pathologies involving the hepato-pancreato-biliary region, including choledocholithiasis, benign strictures of the biliary tract, and hepatic or pancreatic malignancies.72–74 The diagnosis of perihilar cholangiocarcinoma is notoriously difficult and in a patient presenting with obstructive jaundice requires high index of suspicion combined with multiple complementary diagnostic studies.18,74
Serum aminotransferases, alkaline phosphatase, and bilirubin (total, direct) levels are usually the initial laboratory tests obtained in those who present with jaundice or right upper quadrant pain. In patients with perihilar cholangiocarcinoma and complete obstruction of the biliary system, the serum total bilirubin and its direct fraction levels are elevated in conjunction with an increase in alkaline phosphatase level.3,18 The magnitude of this raise in total bilirubin level in patients with perihilar cholangiocarcinoma is higher than what is usually seen in biliary obstruction secondary to benign etiologies (such as choledocholithiasis), with a total bilirubin level of more than 10 mg/dL (with an average of 18 mg/dL) in contrast to 2 to 4 mg/dL. However, isolated elevation of serum alkaline phosphatase level (in the absence of elevated total and direct bilirubin levels) is suggestive of an incomplete obstruction of the right or left hepatic ducts. In these patients, elevated levels of 5′-nucleotidase and γ-glutamyl transpeptidase confirm the hepatobiliary origin of the excess alkaline phosphatase.
Transaminase levels (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) are initially normal in patients with perihilar cholangiocarcinoma. However, in the setting of long-standing biliary obstruction and resulting cholestatic hepatocellular injury, the aminotransferases along with other indicators of liver function such as prothrombin time (PT)/international normalized ratio (INR) might increase. Additionally, prolonged obstruction of the common bile duct can result in malabsorption of fat-soluble vitamins (A, D, E, K) resulting in an elevated PT/INR.3 Occasionally, hypercalcemia may be seen in the absence of metastatic disease.75 Nonspecific markers of systemic malignancy (such as albumin and lactate dehydrogenase LDH) might be altered in advanced stages of the cholangiocarcinoma.
Tumor markers are attractive diagnostic tools because of the ease in obtaining samples and their relatively low cost. Carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) are two markers being used broadly in diagnosis and surveillance of several intra-abdominal malignancies, and its specificity in the diagnosis of cholangiocarcinoma is limited.76–78 Current United Network for Organ Sharing (UNOS) policy for transplantation uses a cutoff value of ≥100 U/mL for surveillance of cholangiocarcinoma in patients with PSC, although higher values of ≥129 U/mL have been used by other investigators to increase the test’s specificity.79,80 CA 19-9 values > 1000 U/mL are suggestive of advanced disease, often involving the peritoneum.79,81,82 If initially elevated, CA 19-9 can be used to follow the efficacy of treatment and surveillance for recurrence of the disease. Important to note is that the expression of CA 19-9 requires the presence of the Lewis blood group antigen, and in patients with a Lewis-negative phenotype (almost 10% of population) CA 19-9 cannot be detected in the serum.83,84 The serum levels of CA 19-9 should be measured before and after resolution of cholangitis or biliary obstruction to avoid false-positive results.76,85
Carcinoembryonic antigen is a glycoprotein that has been used as a tumor marker for several gastrointestinal malignancies.86 A broad spectrum of benign and malignant diseases can raise the serum levels of CEA and the sensitivity and specificity of this tumor marker in the diagnosis of cholangiocarcinoma is even lower than that of CA 19-9.86 However, if elevated, CEA levels can be useful for following the effect of the treatment and to detect possible disease recurrence.
Carbohydrate antigen 19-9 and CEA have been used in combination to create a diagnostic tumor index (CA 19-9 + [CEA × 40], with diagnostic cutoff value of >400); however, while this initially appeared to have higher sensitivity and specificity for diagnosis of cholangiocarcinoma,86,87 the results could not be reproduced and currently its utility is unclear.88 Several other tumor markers such as CA 50, CA 195, DU-PAN-2, CYFRA 21-1, and RACAS1 have also been studied3,89; however, none of these has been shown to have sufficient diagnostic accuracy to be useful in the identification of cholangiocarcinoma. Serum and bile levels of IgG4 should be obtained to rule out IgG4-related cholangiopathy, although elevated levels of IgG4 has been detected in cholangiocarcinoma.90,91
The radiological workup of cholangiocarcinoma relies upon the accurate staging of bile duct and vascular involvement, the background liver, as well as detection of extrahepatic disease. Each imaging modality has unique benefits and role. Table 133-1 summarizes the features of each, which are further described in detail below. Imaging should be performed before any type of biliary drainage.92,93 Biliary duct stent placement results in mild inflammation of bile duct, which is indistinguishable from superficial spread of cholangiocarcinoma, and overestimation of tumor extent. Background liver steatosis, cirrhosis, and evidence of portal hypertension should be noted as part of surgical planning in instances where major hepatectomy is considered. Figures 133-2 to 133-8 detail clinical scenarios with accompanying images to demonstrate the key findings in cholangiocarcinoma, using the following radiologic modalities.
Imaging Modalities for Diagnosis/Staging of Cholangiocarcinoma
| Modality | Benefits | Drawbacks | Special Information |
|---|---|---|---|
| US |
|
|
|
| CT |
|
|
|
| MRI/MRCP |
|
|
|
FIGURE 133-2
Sixty-five-year-old woman with hilar cholangiocarcinoma. Contrast-enhanced CT images in coronal (A), sagittal (B), and transverse (C) planes show biliary duct dilation secondary to a hilar cholangiocarcinoma. The relationship of tumor (white arrow) to vasculature is essential for determining resectability. Note the replaced right hepatic artery (arrow heads) travels under the right portal vein (black arrow) and comes very near the inferior margin of the tumor, best appreciated in sagittal plane.
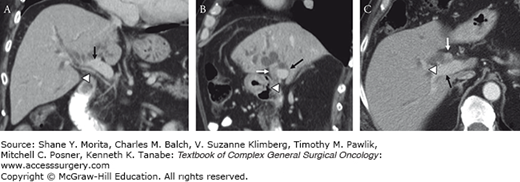
FIGURE 133-3
Images from the same patient as in the preceding figure demonstrate the biliary anatomy relative to tumor involvement. MRCP (A), endoscopic retrograde cholangiopancreatography (ERCP) (B), and percutaneous transhepatic cholangiography (PTC) (C) images show a bismuth IV tumor involving the right and left hepatic ducts. Note that the MRCP was performed prior to decompression, which allows for better depiction of the ducts and regions of stricturing in a noninvasive manner. The right ductal system could not be accessed during ERCP, and subsequent PTC was performed to decompress and alleviate jaundice while the patient underwent workup for possible resection.
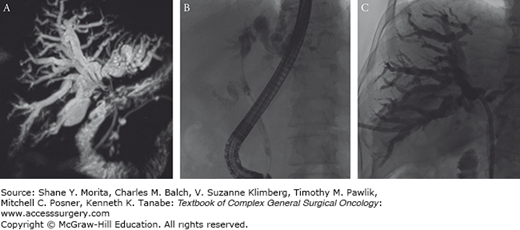
FIGURE 133-4
Same patient from Figs. 133-2 and 133-3. Transverse CT and MRI images show the distribution of ductal dilation and associated atrophy of the left hemiliver. A, B. Note the concave capsular margin compatible with mild capsular retraction from underlying fibrosis and volume loss (arrows). C, D. Volumetric assessment of the liver shows the contours of the left and right liver outlined in orange and blue, respectively. Accurate presurgical volumetrics are necessary for assessing adequate functional liver reserve prior to major hepatectomy.
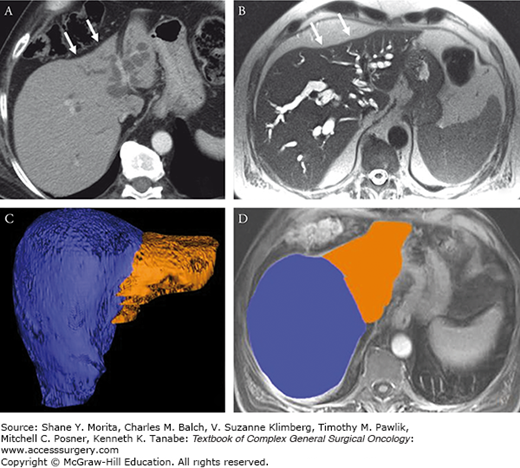
FIGURE 133-5
Seventy-three-year-old man with hilar cholangiocarcinoma. Portal venous phase postcontrast MRI (A) and T2-weighted MRI (B) show a periductal infiltrating lesion manifest by ductal thickening and enhancement (arrows) without a discrete mass. The right hepatic artery (black arrows) (C) abutted the posterior margin of the tumor. MRCP maximum intensity projection image (D) shows the extent of stricturing involving the common hepatic, right and left ducts.
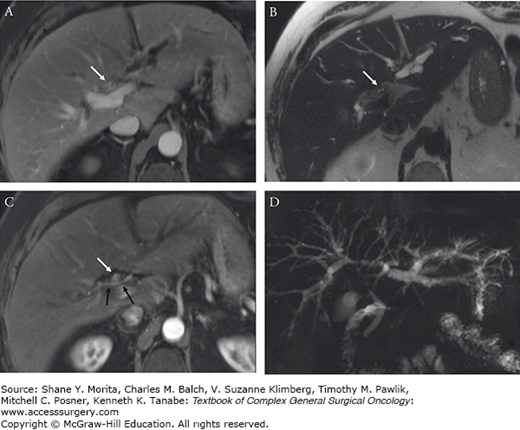
FIGURE 133-6
Same patient from Fig. 133-5 underwent right portal vein embolization (PVE) (A) as a hypertrophy procedure prior to extended right trisectionectomy. Transverse portal venous phase MRI (B) obtained 4 weeks following PVE showed adequate hypertrophy of the left lateral section enlarging from 21% future liver remnant (FLR) on pre-PVE imaging to 31% FLR on follow-up imaging. Postoperative CT image shows the remnant liver (C).
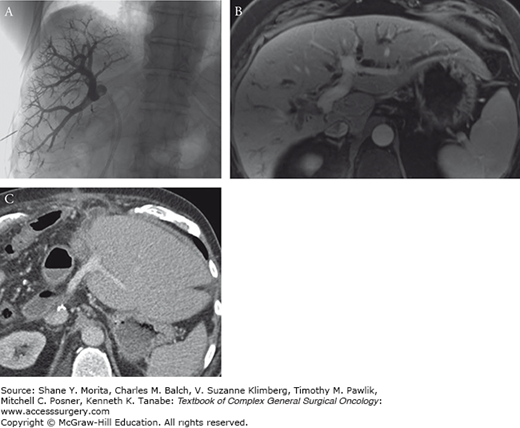
FIGURE 133-7
Sixty-four-year-old woman with a left lateral section intrahepatic mass-forming cholangiocarcinoma. Transverse contrast-enhanced MRI shows a large lesion in the lateral section. Transverse contrast-enhanced CT image (A) shows a large mass (black arrow) which abuts the middle hepatic vein (arrow). The right hepatic vein (arrowhead) is free with a large margin of tissue separating it from the lesion. A slightly more caudal image (B) shows the relationship of tumor to the main and right portal vein (arrows). The bright white bile duct stent is nicely demonstrated relative to the tumor (arrow) and portal vein branches on the maximum intensity projection from the CT (C). MRCP image shows dilated peripheral branches of left duct but no involvement of the right hepatic duct (D). Given the extensive size of tumor and complex resection, the patient also underwent a PET/CT to ensure no distant metastases were present (E).
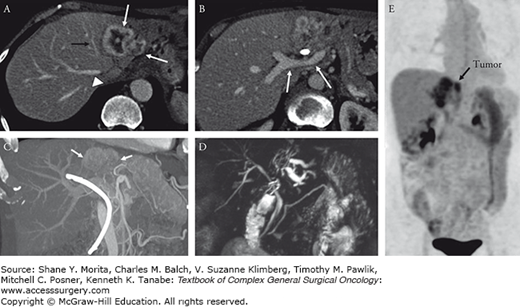
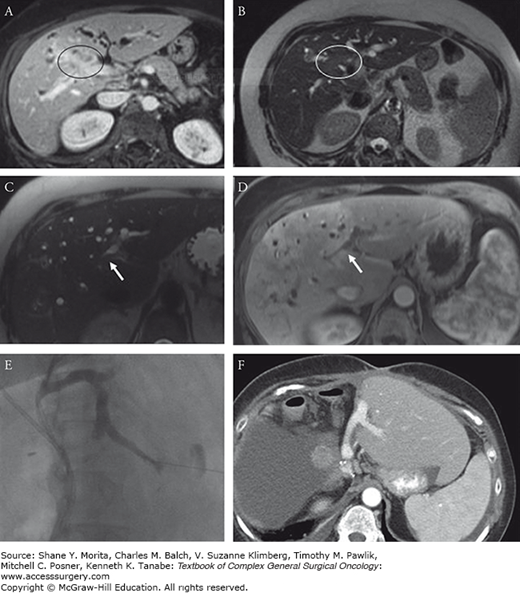
FIGURE 133-8
Sixty-one-year-old woman with a hilar cholangiocarcinoma. Transverse contrast-enhanced (A) and T2-weighted (B) MRI images show a mass centered upon the right liver pedicle (circled region). The patient underwent portal vein embolization and stenting to prepare for possible right trisectionectomy. On follow-up imaging there was potential extension of tumor along the left lateral section duct above the level of the stent (arrows) (C, D). To further decompress the lateral section and help resolve this as inflammation versus a malignant stricture, a percutaneous biliary injection/drainage was performed (E) which showed no malignant stricture. The patient went on to have an extended right trisectionectomy (F).
A noninvasive cost-effective modality, transabdominal ultrasonography (US) is usually the first diagnostic imaging study requested in initial investigation of patients with hepato-pancreato-biliary complaints (including jaundice and right upper quadrant abdominal pain).94 However, US findings are largely operator-dependent and play a minimal role in the definitive diagnosis of cholangiocarcinoma.95 US can be used to exclude underlying benign pathologies (such as cholelithiasis, cholecystitis) as the cause of presenting symptoms. In addition, US is sensitive in detecting biliary tract dilation that can be seen in cholangiocarcinoma and in localizing the level and assessing the severity of the obstruction.94,96 Ductal dilation greater than 6 mm in the absence of bile duct stones is suggestive of an obstructing bile duct lesion.94,96 In perihilar cholangiocarcinomas, the intrahepatic biliary ducts will be typically dilated with normal-caliber extrahepatic ducts and decompressed gallbladder.94,97 Occasionally a discrete smooth hilar mass or a polypoid intraluminal lesion might be visible in the cases of nodular cholangiocarcinoma or papillary tumors, respectively.97 However, the more common scenario is that of intrahepatic ductal dilation without a clearly discerned mass on US. In some instances it may be useful to use duplex imaging (color Doppler) to evaluate the major vessels (portal vein and hepatic artery) involvement by the tumor in cases where there is a specific question.98
Multidetector-row computed tomography (MDCT) is less operator-dependent and more reproducible from center to center. The proper protocol for diagnosing hepato-pancreato-biliary pathologies by MDCT is with a triple-phase postcontrast technique with sufficiently thin slices (1 to 3 mm thick). Triple-phase CT acquires images in the arterial phase, portal venous phase, and delayed phase allowing for optimal assessment of lymphadenopathy, vascular involvement, and extrahepatic disease.99,100 With state-of-the art MDCT available in most centers, the application of MDCT in the diagnosis of perihilar cholangiocarcinoma has increased.101,102 MDCT accuracy for the detection of vascular involvement (either portal vein or hepatic artery) has been reported to be as high as 90%.102–104 MDCT is also useful in identifying the longitudinal extension of the tumor, an important factor when assessing resectability.101,102,105 Moreover, MDCT is useful in detecting the presence of lobar atrophy, which is usually seen as a result of prolonged obstruction of the ducts and/or portal pedicle.99 Obstructive jaundice is a late presentation and the presence of intrahepatic biliary duct dilatation and lobar or segmental liver atrophy also suggests the site of the tumor origin. In addition, an atrophic liver lobe in the presence of hypertrophic contralateral lobe (the atrophy–hypertrophy complex) and intrahepatic biliary duct dilatation may indicate portal vein branch invasion.69 One of the major limitations of MDCT is their low sensitivity in the detection of lymph node (54%) and peritoneal metastasis.100,102,106
Magnetic resonance imaging/cholangiopancreatography (MRI/MRCP) provides similar information to CT about the location of the tumor, lymph node involvement, vascular invasion, hepatic parenchyma, and metastasis, but with greater sensitivity.107,108 Another advantage of MRCP over CT is its ability to depict the biliary tree non-invasively with maximum intensity projections and 3D reconstructions. This is particularly useful in the setting of hilar tumors, where ERCP may not provide sufficient opacification of the bile ducts peripheral to the tumor.108,109 The accuracy of the information provided by MRCP is comparable to that obtained by endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC) without the risk associated with instrumentation of bile ducts and contrast injection (including cholangitis and pancreatitis).92,107,109,110 Moreover, MRI/MRCP enables the surgeon to evaluate the local extension of the tumor and vascular involvement and liver infiltration. MRI/MRCP is currently considered the best initial investigation in patients with suspected cholangiocarcinoma.2,109
Fluorodeoxyglucose-positron emission tomography (FDG-PET) and positron emission tomography-computed tomography (PET-CT) have a role in select patients with cholangiocarcinoma with several limitations. FDG-PET, and recently integrated PET-CT, especially has limited value in initial staging of the tumor; however, it can aid in ruling out distant metastasis, and the presence of uptake in lymph nodes may also improve specificity of staging, given the limitations of size/morphology criteria on CT and MRI alone.114–117 In combination with CT or MRI, it can be used to clarify anatomic localization of uptake. Caution must be exercised in interpretation, as false-positives can occur as a result of biliary tract inflammation, infection, or underlying PSC.116–121
Endoscopically, there are four modalities available to aid in the diagnosis of cholangiocarcinoma. These diagnostic modalities include ERCP, endoscopic ultrasonography (EUS), intraductal ultrasonography (IDUS), and cholangioscopy. The endoscopic management of cholangiocarcinoma, which is typically confined to stent placement for palliative biliary decompression or as a preoperative measure used as a bridge to surgery, will be reviewed in the treatment section.
Visualization of the biliary anatomy by direct injection of radiographic contrast material into the biliary system, either by endoscopy (ERCP) or via percutaneous approach (PTC), has been largely replaced by less invasive diagnostic modalities such as CT scan and MRI/MRCP.94,95 However, the main advantage of these invasive modalities is their capability in offering the diagnostic (brush cytology, biopsy, bile sampling) and therapeutic interventions (biliary decompression and stent placement).96 The choice between PTC and ERCP depends on the level of the available clinical and radiologic expertise and the location of the tumor.3,97
While it can be challenging to determine benign versus malignant nature of a stricture using cholangiography alone, the presence of hilar stricture with a length greater than 1 cm, irregular contour, and abrupt transition from normal to stricture is suggestive of malignancy.98 A major risk of direct cholangiographic techniques is cholangitis, with a reported morbidity rate up to 7% and mortality rate up to 1%.99 Adequate drainage of biliary system with stent placement might aid in decreasing the risk of cholangitis after biliary tract manipulations.100 A major limitation of this approach is the inability to assess the extent of infiltrative growth pattern along the duct which may lead to underestimation of the stage.1,101 However, in cases of intraductal papillary type tumors, due to intra-ductal necrotic material, involvement of bile ducts and hence the staging of the tumor will often be overestimated.101
In addition to its high sensitivity in localizing the bile duct lesions and detecting vascular involvement, EUS avoids biliary contamination and sepsis can occur during implementation of direct cholangiography techniques.102 EUS is high-resolution imaging and can detect lesions 3 mm or greater.103,104 It additionally offers the ability to sample suspicious lymph nodes for metastatic involvement. EUS-guided fine-needle aspiration (FNA) or core-needle biopsy of the tumor or regional lymph nodes has a greater sensitivity for diagnosis of cholangiocarcinoma than that of ERCP and brush cytology.92,93,100,105 Diagnostic and staging value of EUS for distal biliary lesions is higher than for proximal lesions.92,103,106 The ability of EUS to evaluate proximal lesions is limited and for the evaluation of proximal biliary system and adjacent structures (such as portal vein, hepatic artery) IDUS is preferred, offering a higher sensitivity with a reported diagnostic accuracy of close to 100% in detecting early biliary duct lesions, local tumor extension, and major vessels invasion.107–110 Although IDUS cannot alone determine benign versus malignant biliary strictures, combining IDUS with other diagnostic modalities (such as MRI and ERCP) can increase their accuracy to this end.111–113 However, IDUS cannot be used to perform FNA tissue sampling.
A small cholangioscope can be used to directly visualize the biliary tract during ERCP or more commonly during percutaneous transhepatic cholangiography.122–124 Although the characteristics of lesions found on cholangiography that suggest malignancy have not been well defined, the presence of mucosal ulcerations, irregular mucosa, asymmetric stricture, and irregularly dilated and tortuous blood vessels is suggestive of malignant lesion.125 Direct tissue biopsy and brush cytology can also be obtained during cholangioscopy.126,127
One of the major limitations of the conventional cholangioscope, besides being fragile and difficult to use, is that its operation requires two endoscopists (one to operate the endoscope and other one for cholangioscope). Recently, a single-operator peroral cholangiopancreatoscopy system known as Spyglass has been developed.128,129







