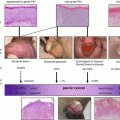
Fig. 3.1
Superficial SCC suitable for total glans resurfacing
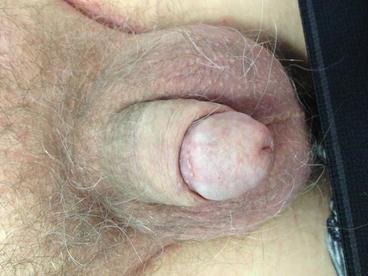
Fig. 3.2
Three months post total glans resurfacing
The procedure is performed under general anaesthesia with pre-operative antibiotics and the use of a penile tourniquet for haemostasis. All epithelium and subepithelium of the glans are marked leaving only a perimeatal and circumcoronal margin. This is undertaken in quadrants starting from the meatus to the coronal sulcus for each quadrant. Deep biopsies from the corpus spongiosum are taken for separate frozen section analysis to exclude invasive disease and confirm complete excision. A split-thickness graft is harvested from the thigh with an air dermatome and should be between 0.008 and 0.016 in. in thickness. The graft is sutured and quilted to the denuded glans with 5-0 interrupted vicryl sutures and dressed with soft paraffin gauze followed by foam dressing to help protect and immobilize the graft. A catheter (14 French silicone) should be placed to keep the wound clean and dry, and the patient is placed on 48-h strict bed rest. The dressing and catheter should be removed on the fifth day post-operatively when the patient is discharged.
An advantage of this surgical approach is its combined diagnostic and therapeutic ability. As well as completely removing the diseased epithelium, TGR also allows more accurate histopathological staging compared to incisional biopsies which are used alongside previously described non-surgical therapies. Shabbir et al. reported that 10 of 25 patients (40 %) who underwent either total or partial glans resurfacing (PGR) had evidence of invasive carcinoma on the final pathological specimens despite all 25 patients having pre-operative incisional biopsies showing Tis only [20]. This finding raises concerns as the majority of patients diagnosed with glanular Tis are primarily treated based on pathology from incisional biopsies and therefore may be under-treated by topical chemotherapy or laser therapy. This is a possible explanation for the higher recurrence rate with laser therapy (26 %) compared to surgical excision/resection (0–4 %) [12, 19–21] (Table 3.1).
Table 3.1
Oncological outcomes following glans resurfacing
Study | Treatment | Patients (T stage) | Patients | Reported outcomes | Mean follow-up (months) |
|---|---|---|---|---|---|
Hadway et al. [19] | TGR | Tis | 10 | No recurrence/progression | 30 |
Shabbir et al. [20] | TGR/PGR | Tis | 25 | Recurrence 4 %, no progression | 29 |
Ayres et al. [21] | TGR | T1a | 36 | Early revision rate 8 % | 21 |
Local recurrence rate 6 % | |||||
Shabbir et al. [20] | TGR (5) | T1a | 7 | No recurrence or progression | 29 |
PGR (2) |
TGR allows optimal preservation of penile length, form, and function, and a number of studies have reported good functional and cosmetic outcome results. Shabbir et al. reported 96 % total graft uptake in a study of 25 patients and another series of 10 patients experienced a 100 % graft uptake [19, 20]. Neither authors reported any postoperative complications nor were there any cases of recurrence following TGR. In another series of ten patients treated with TGR for recurrent, refractory, or extensive disease, there was no evidence of disease recurrence after a mean follow-up of 30 months and over 80 % were sexually active within 3 months of surgery [10].
Partial Glans Resurfacing
For Isolated Foci of Tis/Ta Affecting < 50 % of Glans
PGR is an alternative to TGR in the absence of multifocal tumour, if glanular involvement is below 50 % and only for tumours up to Ta (Figures 3.3 & 3.4). PGR applies the same principles as TGR but has the inherent benefit of conserving normal glans skin which offers increased preservation of sensation and better cosmesis. PGR is associated with a high risk of positive surgical margins, and a sub-analysis of one study found a 67 % positive margin rate in this cohort. Forty percent of men in the PGR cohort required further surgical intervention, but there were still no cases of recurrence or progression over a mean follow-up of 29 months [20]. Another study with 36 patients similarly cited a low recurrence rate of 6 % [21]. Evidence suggests that, where possible, PGR may be a more attractive option for young, sexually active men although all should be counselled that further surgical intervention may be required, which may be in the form of TGR or glansectomy.
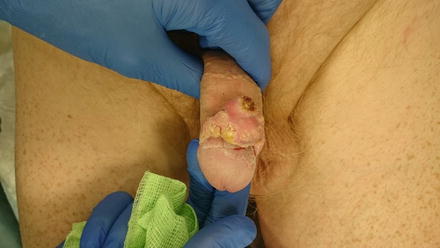
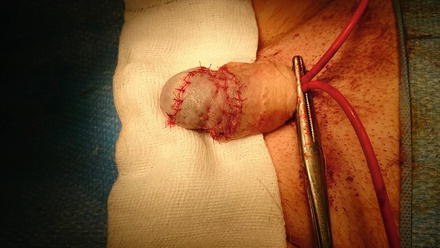

Fig. 3.3
Superficial inner prepuce tumour with differentiated penile intraepithelial neoplasia (PeIN) extending onto the glans, suitable for partials glans resurfacing

Fig. 3.4
Intra-operative: after partial glans resurfacing and circumcision
Moh’s Micrographic Surgery
For Stage T1a, But Has Been Used Up to Stage T3
Moh’s Micrographic surgery (MMS) i s a form of microscopically controlled chemotherapy which can be performed under local anaesthetic. This technique involves removing the entire abnormal lesion in thin sections, with concurrent histological examination using frozen sections to ensure clear margins microscopically [22]. MMS allows maximal preservation of normal penile tissue but is limited by being time-consuming, technically challenging, requiring both a surgeon and pathologist trained in the technique and high recurrence rates. These limitations have meant MMS is used infrequently in centres managing penile cancer. A study by Shindel et al. found a local recurrence rate of 32 %, and Moh himself acknowledging that distal tumours had better cure rates compared to just 57 % in cancers with penile shaft involvement [22, 23]. Due to the above limitations, MMS still remains an infrequently used intervention.
Wide Local Excision/Partial Glansectomy
For Small, Discrete Lesions on the Glans Up to Stage T1a
In the majority of patients, if the defect is small, primary closure results only in minimal glans deformity. In those with larger lesions, or those in close proximity to the urethral meatus, a split skin graft or shaft skin advancement may be required to achieve a good cosmetic and functional outcome.
Partial glansectomy and TGR are best suited to patients with ‘low risk’ T1a disease (G1/G2 disease, with no evidence of lymphovascular invasion). It is vital that patients are compliant and adhere to a close surveillance programme following surgery to ensure early detection of recurrences. Local recurrence or positive surgical margins should be treated by total glansectomy. Early detection with subsequent ‘salvage’ surgery has a high success rate, with no adverse impact on disease survival [24, 25].
Glansectomy and Reconstruction
For Stage T1b/T2 Tumours Confined to the Glans
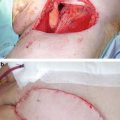
Glansectomy offers the best surgical approach for T2 or high grade T1 disease (Figures 3.5 & 3.6). This procedure utilizes knowledge of the anatomical planes between the corpora cavernosa and corpus spongiosum initially described by Austoni and colleagues [26]. In this procedure the glans is separated from the corpora cavernosa with subsequent formation of a new urethral meatus at the tip of the shaft.
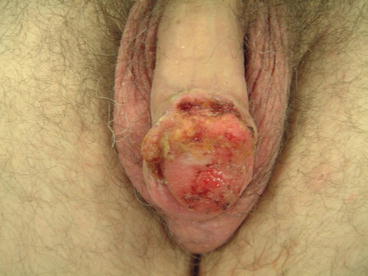


Fig. 3.5
T2a SCC of glans spongiosum, suitable for glansectomy

Fig. 3.6
Post-operative appearance at 3 months’ post glansectomy
A circumferential incision is made in the distal shaft skin (approximately 1 cm below the corona) down to Buck’s fascia. Depending on the location and extension of the penile lesion, a plane of dissection is created to separate the glans from the underlying corporal heads. The urethra is transected and frozen-sections from the corpora and distal urethra can be taken if there are concerns about positive margins. If a positive margin is identified on frozen section, the ‘shaving’ of the corporal heads should be performed prior to mobilization of the urethra or reconstruction.
In situations when the urethra is too ventrally positioned, spatulation and mobilization of the urethra may be required. Shaft skin is advanced to 2–3 cm distal to the tip of the penis and the neoglans created using a split thickness skin graft. Similar to technique for TGR, the graft is harvested from the thigh with an air dermatome and should be between 0.008 and 0.016 in. in thickness. It is sutured and quilted with 5-0 interrupted vicryl sutures and dressed with soft paraffin gauze and a foam dressing. A catheter is placed to keep the graft-site clean and dry. Following 48 h of bed-rest, the dressings and catheter are removed on the fifth post-operative day prior to discharge.
Complications following this procedure include graft failure, graft stenosis, and urethral stenosis but have a relatively low incidence (8 % in a study of 25 patients) [27]. Patients also often have high risk invasive cancer therefore positive margins are not uncommon. Despite this, Veeratterpillay et al. cited only a 13 % positive margin rate and a 4 % recurrence rate in their cohort of 46 patients [28]. Morelli et al. reported their experience with glansectomy and reconstruction in 15 patients with tumours confined to the glans. At a mean follow-up of 36 months, all patients were disease-free, with no cases of local recurrence. All patients were sexually active 2–6 months post-operatively, but all reported reduced glans sensitivity [29]. In the largest series to date of 72 patients who underwent glansectomy and reconstruction, only three local recurrences (4 %) were reported (Table 3.2).
Table 3.2
Oncological outcomes following glansectomy (partial/total)
Study | Procedure | Tumour stage | Patients (number) | Local recurrence rate (%) | Mean follow-up (months) |
|---|---|---|---|---|---|
Pietrzak et al. [33] | Partial/total glansectomy | Ta-T3 | 39 | 2.5 | 16 |
Brown et al. [34] | Partial/total glansectomy | T1-T2 | 5 | 0 | 12 |
Gulino et al. [35] | Partial/total glansectomy | Ta-T3 | 14 | 0 | 13 |
Smith et al. [36] | Partial/total glansectomy | T1-T2 | 72 | 4 | 27 |
Palminteri et al. [37] | Partial/total glansectomy | T1-T2 | 12 | 0 | 32
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|