Preoperative Evaluation and Preparation
Pain is a common presentation in patients presenting with isolated pelvic disease, who may be relatively young and otherwise healthy. With respect to the risks of radical surgery though, this patient may have already undergone several rounds of surgery, radiation, and chemotherapy. The main objectives of the preoperative evaluation are to determine resectability, exclude metastasis, and assess the patient’s general medical status. Radical pelvic surgery imposes a heavy burden on the reserves of various organ systems. Hence, a thorough assessment of the severity of co-morbidities including diabetes, cardiovascular disease, pulmonary, hepatic, and renal insufficiencies is of vital importance. Given the potential postoperative sequelae requiring significant rehabilitation, evaluation of psychologic status, psychosocial support system, and preoperative teaching are also critical. These factors in patient selection have been addressed in detail elsewhere.6–8,15,17,22–26 A complete clinical examination should include documentation of baseline neuromuscular function of the lower extremities, paying particular attention to any deficits caused by the tumor. Rectal and vaginal examinations if possible are helpful in assessing the extent of the tumor, its fixation to the lateral wall, and possible involvement of anterior pelvic viscera.
Essential imaging studies include a computed tomography (CT) scan of the abdomen and pelvis, plain X-rays of the chest and lumbosacral spine, a bone scan, and magnetic resonance imaging (MRI). Aplain chest radiograph is usually sufficient to rule out lung metastasis, although a chest CT scan may be required in the presence of suspicious but equivocal findings. The CT scan demonstrates the local extent of the tumor and may also reveal enlarged periaortic nodes and liver metastases. A bone scan and possibly a positive-radio-nuclear scan (PET scan) are important to exclude marrow involvement. In the presence of a positive PET scan, an MRI may help define whether there is anatomic marrow invasion. MRI T2-weighted sagittal views are helpful in planning operative strategy by delineating the relationship of the tumor to the sacrum and especially in demonstrating the upper limits of the tumor. If suspicious areas are seen on imaging, CT-guided needle biopsy can provide tissue diagnosis. In some cases of primary sacral cancer, an open biopsy may be necessary. Laparoscopy provides direct evaluation of peritoneal metastases. Arteriography is selectively performed only with extensive tumors. In patients with rectal carcinoma, evaluation of the colon with colonoscopy (via rectum or colostomy) or contrast enema is important for detection of synchronous lesions which would alter the extent of the resection. Cystoscopy is useful in evaluating the extent of urinary bladder involvement and the finding of limited dome involvement may allow for a partial cystectomy with preservation of function, whereas invasion of the trigone will undoubtedly require total cystectomy with urinary diversion.
Regarding nonsurgical treatment, patients with large primary sarcomas are candidates for preoperative radiation or chemotherapy, whereas those with chordomas usually receive postoperative radiation. For sarcomas, we use 3000 to 3500 cGy of radiation, given in 10 to 15 fractions and doxorubicin, 30 mg/day for 3 days, given by continuous infusion, as a radiation sensitizer. Patients with locally advanced or recurrent rectal adenocarcinomas or epidermoid anorectal carcinomas also receive preoperative chemoradiation, unless they have previously undergone radiation therapy. For rectal adenocarcinomas, 45 to 50 Gy of radiation is administered in 20–25 fractions along with sensitizing 5-fluorouracil, 750 mg/m2/day for 5 days or 250 mg/ M2/d by continuous infusion during the period of radiation. Patients with advanced epidermoid carcinoma receive preoperatively up to 50 Gy of radiation in 15 fractions plus 5-fluorouracil, 750 mg/m2/day for 5 days and mitomycin, 15 mg/m2/day for 1 day.
Patients who still have the rectum in continuity with the gastrointestinal tract are instructed to change their diet to clear liquids 48 hours before the operation. The day before surgery, standard mechanical bowel preparation begins with the drinking of a standard solution until the rectal effluent becomes clear. Two doses of antibiotics, neomycin and metronidazole, are also given orally. One must guard against fluid and electrolyte losses during bowel preparation by ensuring adequate oral intake or with supplementary intravenous fluid. A broad spectrum antibiotic with activity against gram-negative and anaerobic bacteria is given parenterally just prior to incision.
 Operative Technique
Operative Technique
Chapter 11 describes the surgical strategies for primary or recurrent pelvic malignancy. Chapter 10 considers the abdominoinguinal approach for pelvic sidewall tumors. They complement the following description.
 Tumors Primarily Involving Posterior Pelvis or Pelvic Sidewalls
Tumors Primarily Involving Posterior Pelvis or Pelvic Sidewalls
For the purpose of this discussion, the technique of an ASR is presented here because it encompasses most of the problems encountered in managing pelvic cancer. Inclusion of the sacrum can be considered to be the posterior margin of an extended pelvic exenteration. We perform a two-stage procedure including a 24 to 36 hour intermission in the intensive care unit (ICU). Others have described it as a single stage procedure, entirely in the lithotomy position.27 The abdominal stage basically entails determination of resectability, lymph node dissection, urinary and fecal diversion as indicated, and vascular isolation of the tumor. The actual resection is reserved for the sacral stage. The procedure is well detailed in Joel Bauer’s Colorectal Surgery Illustrated.28
For the abdominal stage, the patient is positioned in lithotomy and ureteral stents are placed. Careful positioning is important because of the rare but serious leg complications that can occur especially with procedures of this length.29 The procedure starts with entry into the abdomen through a generous lower midline incision extending from the pubic symphysis to approximately 10 cm above the umbilicus. Ameticulous exploration is done to rule out extrapelvic disease which precludes curative resection and is therefore a contraindication. This includes peritoneal seeding, unresectable metastases to the liver, extra-pelvic lymphadenopathy (i. e., paraaortic or external iliac), or involvement of the sacral promontory.
If no extrapelvic disease is found, exposure of the pelvis is obtained by packing the small bowel up into the middle and upper abdomen with use of a self-retaining retractor. If the rectum is still intact and must be resected, the site of transection in the sigmoid colon is selected by taking into consideration the adequacy of the length and blood supply of the remaining left colon for forming a permanent colostomy without tension or ischemia. The mesocolon is serially clamped, divided, and ligated from the selected transection site to the level of the sacral promontory and the sigmoid is transected with a linear gastrointestinal stapler. The upper rectum is mobilized with sharp dissection. Dense adhesions between the tumor and bowel or bladder should be considered cancerous and necessitate removal of the tumor-attached organ or at least the site of attachment. To avoid jeopardizing a curative resection, dissection must be avoided in the area where the tumor appears to invade the sacrum.
Aortoiliac node dissection is started at the level of the aortic and caval bifurcations and continues along the common and external iliac arteries; it includes the internal iliac, the obturator, and the hypogastric nodes (Fig. 12.1). These lymph nodes are submitted for histopathologic study separately from the main specimen. Extensive nodal involvement in the lower pelvis generally precludes continuing the resection, but the presence of enlarged but easily resectable obturator nodes does not. If the ureters are free of tumor, they are dissected and fixed anteriorly to the lateral pelvic wall just below the external iliac artery and vein to prevent injury during the posterior dissection. If the bladder and distal ureters are involved but considered resectable, the ureters are divided, the bladder is dissected free from the lateral side walls but left attached to the posterior tumor. An ileal conduit is constructed in a standard fashion (Fig. 12.2). An involved uterus or ovaries are treated similarly.
If the tumor extends into the internal iliac artery or vein, these are bisected from the parent vessel (common iliac artery or vein) and attached to the tumor and posterior pelvis (to be resected from above; Fig. 12.3). In general, tumor involvement of the common iliac artery or vein is considered nonresectable for primary or recurrent rectal, anorectal, or gynecologic cancer. For primary sarcoma of the pelvis, however, localized sarcoma demonstrating vascular invasion may be resected by composite resection with reconstruction as dictated by specific circumstances.
Pelvic devascularization is optimized during the abdominal stage because of easier exposure and it facilitates hemostasis during the sacral stage. This is accomplished by transecting and ligating with sutures the internal iliac arteries and veins (Fig. 12.3 ). An effort should be made to ligate the internal iliac artery beyond the first branch to preserve vascularization of the skin and muscle flaps to be used for closure of the posterior wound. The middle sacral artery and vein and individual branches to the tumor mass are divided if found in the plane of dissection. If the bladder is to be preserved it is important to maintain at least one cystic artery. There is also important vascular inflow from retropubic vessels and these attachments should not be disturbed.
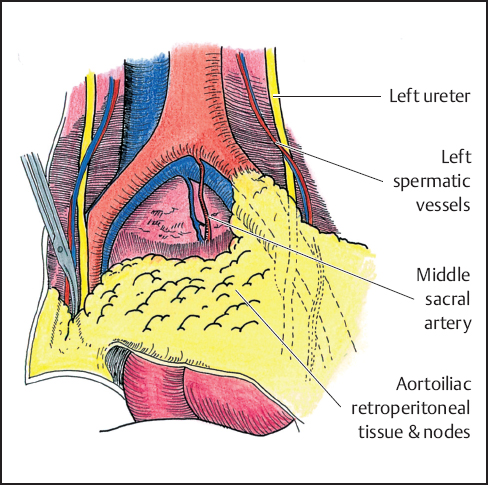
Fig. 12.1 Aortoiliac and obturator node dissection.
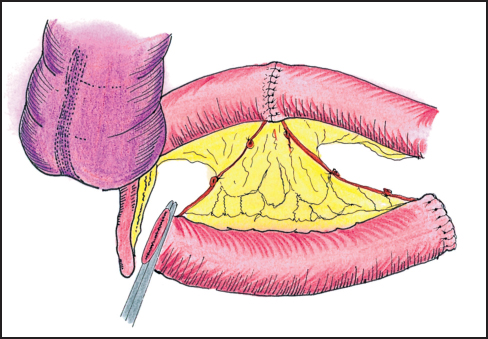
Fig. 12.2 Ileal conduit and ureteral anastomosis.
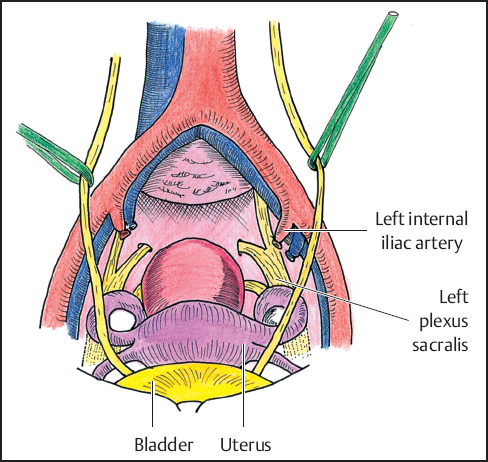
Fig. 12.3 Pelvic devascularization.
The peritoneal floor is restored with Vicryl mesh, preferably with an interposed omental pedicle or local peritoneal flap to protect the bowel from fistula formation (Fig. 12.4).30 A transpelvic rectus abdominis musculocutaneous rotation flap can be used to fill the resulting pelvic dead space and close the perineal wound. This can help minimize healing complications, especially if the area had previously undergone radiotherapy.31 The closed end of the ileal loop is sutured to paravertebral tissues proximal to the periosteum of the sacral promontory to prevent twisting of the mesentery. Before closure of the abdominal incision, the distal end of the ileal loop and the divided end of the sigmoid colon are exteriorized through openings at the predesignated areas of the abdominal wall. The distal ends of the ureteral stents are also brought out through the ileostomy.
After satisfactory hemostasis has been achieved, the abdominal incision is closed. If oozing persists despite meticulous hemostasis, the pelvis is packed with laparotomy sponges, which are removed during the sacral part of the procedure. After closure of the abdominal incision, the stomas are matured with interrupted 4–0 Vicryl sutures. The ureteral stents are transfixed to the skin surrounding the stoma of the ileal loop and left in place for 5 to 7 days; they are removed only after anastomotic integrity has been documented by means of a retrograde contrast study.
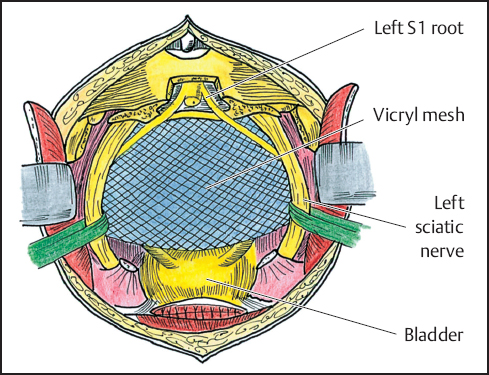
Fig. 12.4 Vicryl mesh, peritoneal, or omental sling is developed to prevent posterior herniation of bowel.
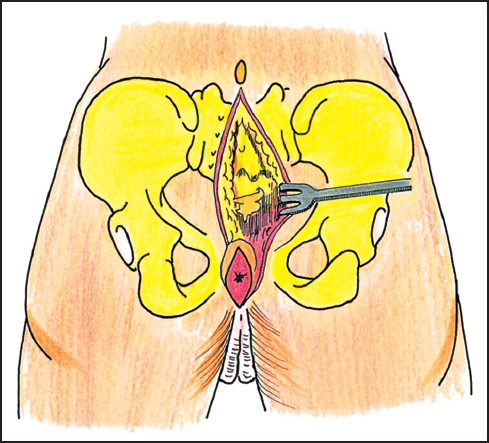
Fig. 12.5 Posterior skin incision.
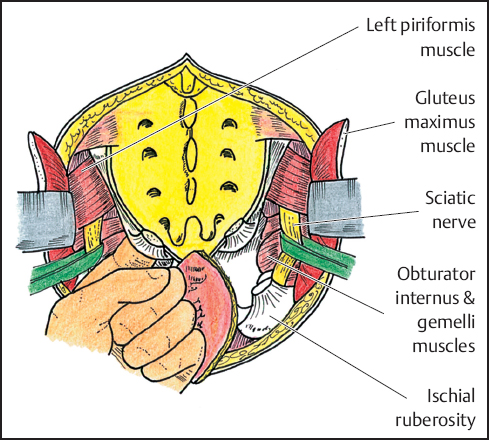
Fig. 12.6 Identification of sciatic nerves. Sacrotuberous and sacrospinous ligaments are incised. Surgeon’s finger “breaks through” endopelvic fascia to assess proper site for sacral osteotomy.
Upon completion of the abdominal stage, the patient is allowed to equilibrate and is to become optimized in the ICU for approximately 24 hours. The sacral stage of the procedure begins with the patient being placed in the prone position. A posterior sacral incision is made from the spinous process of L5 to the perineum. The lower part of the incision has an elliptical course so it can include the anus and urethra, depending on which pelvic organs are to be removed. Full-thickness flaps are raised at the level of the sacral periosteum to the lateral extent of the sacrum (Fig. 12.5). The gluteus maximus and medius muscles are dissected from their sacral origins with maintenance of a fascial cuff on the muscles for the subsequent midline wound closure.
The sciatic nerve is located by retracting the gluteus maximus and underlying piriformis muscles superiorly at the lateral aspect of the middle portion of the sacrum. The nerve lies superficial to the obturator internus and gemelli muscles as it courses inferolaterally midway between the ischial tuberosity and the greater trochanter. During the dissection, it is encircled with a vessel loop for ease of identification (Fig. 12.6). To access the pelvis, the sacrotuberous and sacrospinous ligaments are incised at the level of their attachments to the ischial tuberosity and ischial spine. A finger is then inserted medially to the sciatic nerve and advanced deeply beneath the piriformis muscles and through the underlying endopelvic fascia. With this maneuver, the surgeon “breaks through” the endopelvic fascia to reach the anterior surface of the sacrum in the area of the pelvic dissection performed during the abdominal procedure. This approach directs the subsequent sacral osteotomy and ensures an adequate margin proximal to the tumor. Further dissection is carried out around the sacrum by incising the piriformis muscles and the soft tissues surrounding the sciatic nerve.
A laminectomy is performed at the planned level of sacral transection, usually between L5 and S1, to help dissect the nerve roots and ligate the terminal end of the dural sac. The proximal sacral roots are identified and an effort is made to preserve them by dissecting them free from the portion of the sacrum to be resected. After the resection line on both sides of the sacrum is determined, an osteotome or oscillating saw is used to transect the bone while the surgeon’s finger is positioned anteriorly to protect the intraabdominal contents (Fig. 12.7). For resections above the level of S3, greater effort is required as the line of resection is continued laterally through the sacroiliac joints. Care is taken not to injure the lumbar component of the sciatic nerve. The sacral components of the nerve are dissected if necessary. The sacrum, pelvic sidewall, and tumor-bearing structures are removed en bloc (Fig. 12.8). Hemostasis is obtained after initial packing of the wound to gain temporary control of bleeding. The region is examined carefully and any residual tumor or devitalized tissue is removed. If the presence of residual tumor is suspected, the margins are examined histologically by frozen section. Additional margins are serially obtained until there is frozen section confirmation that margins are free of tumor. When possible, a 2-cm margin is desirable. It is important to examine the specimen carefully with the pathologist. It is also helpful to perform biopsies at the periphery of the resection sites that correspond to high risk areas observed after examination of the specimen.
Closed-suction drains are placed bilaterally and the sacral wound is closed. If the fascial origin of the gluteus maximus has been preserved, the muscles are approximated in the midline with heavy nonabsorbable mono-filament suture, thereby forming a new pelvic floor. A relaxing incision at the level of the lateral insertion of the gluteus maximus on the greater trochanter may be necessary to allow additional medial advancement of the muscle (Fig. 12.9). The subcutaneous tissue is approximated with interrupted absorbable sutures and the skin with staples. Depending on the extent of the resection and the status of the patient, reconstruction with musculocutaneous flaps may be performed at the time of resection or delayed for a few days.
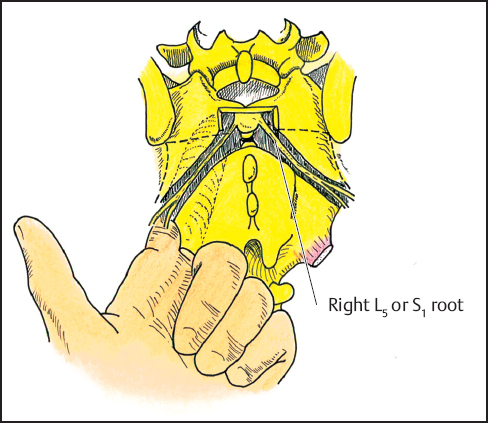
Fig. 12.7 Laminectomy (L5-S1) with dissection and preservation of selected sacral root (usually S1 or more distal if possible).
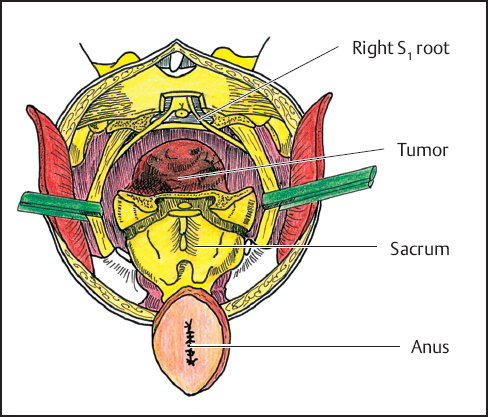
Fig. 12.8 The sacrum, pelvic sidewalls, and tumor are removed en bloc along with the attached structures (bladder, retained rectum) as indicated.
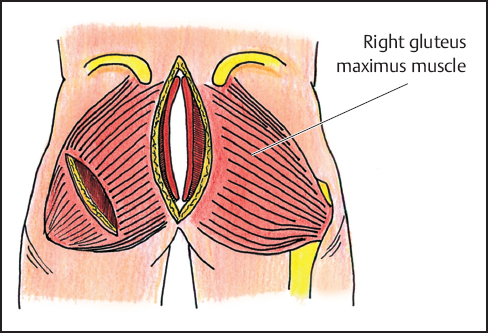
Fig. 12.9 The gluteus maximus muscles are approximated to midline forming a new pelvic floor. To allow greater medial advancement of the muscle, it may be necessary to incise the lateral insertions of the gluteus maximus on the greater trochanter.
 Complications, Mortality and Morbidity
Complications, Mortality and Morbidity
The potential complications of extended radical pelvic operations include the same spectrum of problems occurring after major abdominal surgery. In addition, complications related to the urinary diversion, the stomas, and the sacral resection can arise. However, these patients are at greater risk because they have a healing disadvantage from prior surgery and radiation therapy.
The long anesthesia and operative time coupled with the vast extent of dissection and exposure results in an extensive intravascular volume deficit. Bleeding, third space and insensible fluid losses requiring massive replacement represents a difficult, sometimes overwhelming challenge. It is this stress that matters most in patient selection. Close intraoperative and postoperative hemodynamic monitoring is essential. We usually use autotransfusion intraoperatively. Fatal postoperative hemorrhage has been reported after radical pelvic surgery.22,25,32 Death from uncontrolled bleeding, however, should be preventable with proper handling of the pelvic vasculature and establishment of good hemostasis during the procedure. When satisfactory hemostasis cannot be accomplished, packing of the pelvis, followed by a return to the operating room 2 or 3 days later to remove the pack, is safe and effective. Another potential problem in this setting is the development of abdominal compartment syndrome resulting in respiratory compromise, renal dysfunction, and hemodynamic instability. Emergent decompressive laparotomy is required.
Patients undergoing radical pelvic surgery are also prone to cardiopulmonary complications caused by massive fluid shifts, rapid administration of large volumes of fluid, and substantial continuing postoperative fluid loss.22,23,25,32,33 Close monitoring, with appropriate fluid replacement and correction of electrolyte imbalances plays a critical part in preventing cardiac problems. A sufficient urine output indicates not only the adequacy of hydration and renal function but also the patency and integrity of the ureteral anastomosis.
Wound infections have been an important cause of morbidity, particularly in earlier series.6,22,23,25,33 Infections and flap separation after ASR occur markedly more often in patients undergoing the procedure for recurrent disease and in those who had received high doses of radiation preoperatively. Large open granulating wounds contribute to sepsis and volume deficit. Pelvic abscesses and collections such as lymphoceles and urinomas should be initially managed nonoperatively with percutaneous drainage.
Intestinal obstruction is a common early postoperative complication that may cause considerable morbidity if it is not diagnosed quickly and treated properly.6,22,23,25,33 The principal predisposing factors are the empty, deperitonealized pelvis and the presence and position of the urinary-diversion conduit.22,24,26,34 The empty pelvis is also the source of enteroperineal fistulas.24,25–37 Delay in the surgical management of early postoperative intestinal obstruction runs the risk of entering the pelvis after the formation of adhesions which increases surgical mortality and morbidity.38 In such situations,sound surgical judgment dictates that if the bowel cannot be dissected free of the pelvis safely, the obstruction should be relieved by an intestinal bypass procedure.22,36,37,39 Preoperative radiotherapy facilitates local control at the expense of morbidity associated with operating on irradiated tissue. Because the incidence of intestinal obstruction is significantly higher in patients given preoperative irradiation, particularly in those without pelvic reconstruction, it is advisable to perform pelvic reconstruction with use of omental or myocutaneous flaps or colonic advancement in these patients.24,26 Immediate or delayed reconstruction by mucocutaneous flaps is associated with fewer healing complications than primary closure in this subset of patients that have undergone radical pelvic surgery or received preoperative radiation.40 Similarly, obliteration of the pelvic defect should be done in patients likely to undergo postoperative radiation to prevent descent of the small bowel into the pelvis where it will be exposed to the radiation.41 Gracilis, rectus abdominis, tensor fascia lata, and inferior gluteal flaps have all been used successfully to fill pelvic defects.42–44 The potential for reducing pelvic adhesions by barrier agents such as Interceed/GoreTex is an additional consideration that merits exploration.
The lower urinary tract is vulnerable during pelvic surgery depending on the extent and proximity of dissection, preoperative radiotherapy, and extent of disease. Ureteral stents do not prevent these injuries, but do aid in early recognition and thus prompt correction. Dehiscence of intestinal and ureteral anastomoses has been reported22,23,33 and appears to be more frequent after preoperative radiation. It can be minimized by using nonirradiated ileum or colon for formation of the ileal conduit.22
Other early complications that have been reported include fecal and urinary fistulas, urinary tract infections, hydronephrosis, retraction or separation of the stomas, thrombophlebitis, pulmonary embolism, evisceration, psychosis, cerebrovascular accident, prolonged ileus, atelectasis, and pneumonia.16,22,23,25,28,33 Lower extremity complications such as deep venous thrombosis, perineal neuropathy, and compartment syndrome are rare but serious complications of prolonged pelvic surgery in the lithotomy position.28
Late complicationsoccur quite often, making evident the importance of continued surgical follow-up. Late intestinal obstruction and enteroperineal fistula formation have been reported and surgical management of these problems entails a considerable risk.22 The surgical therapy used is the same as that for early similar complications. Again, if the risk associated with dissection of the small intestines from the pelvis is high, an intestinal bypass procedure should be done. Although it is unlikely that an enteroperineal fistula will close after a bypass procedure, leaving a draining fistula in the perineum may be the proper choice if the risk involved in dissecting the small intestine from the pelvis is prohibitive.22 Extensive resections of the small bowel should be avoided because they may lead to short bowel syndrome.
Pyelonephritis occurs as a late complication and is usually easily controlled with the proper antibiotics if there is no mechanical obstruction at the ureteral anastomosis that is causing stasis. If obstruction is present, it should be corrected surgically. Extensive dissection of the ureter in patients who have undergone radiation therapy incurs the risk of ischemic damage to the ureter and possible resultant ureteral fibrosis or fistulas. Acute episodes of ureteral obstruction in such patients may initially be managed by percutaneous nephrostomy to permit controlled urinary decompression; this allows time to assess the problem and plan electivereconstruction.
Other late complications include perineal, paracolostomy and incisional hernias.22,25 They are usually amenable to surgical repair with use of conventional techniques, synthetic mesh or myocutaneous flaps.22,43,45,46
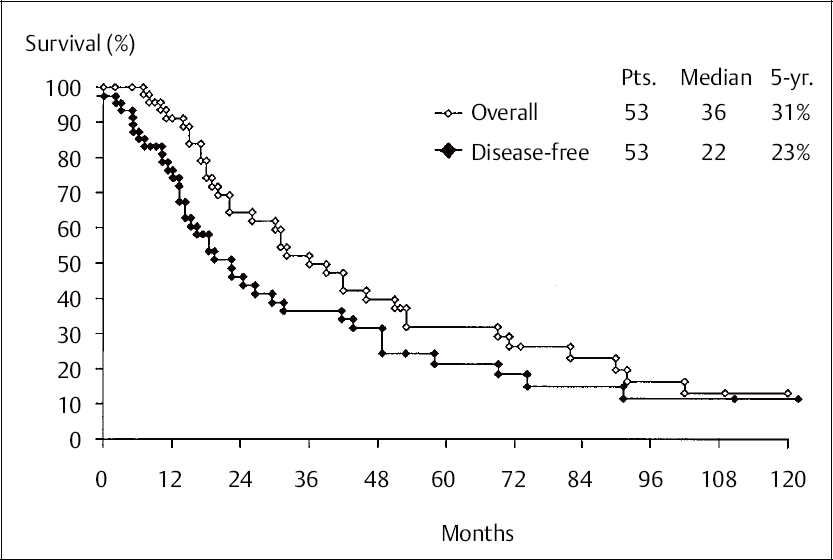
Fig. 12.10 Overall and disease-free survival compared in 53 patients treated for recurrence by abdominosacral resection. Pts: number of patients; median: median survival time; 5-yr: five-year survival.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


