Study
N
OS
CSS
Follow-up (months)
Lont et al. [6]
25
5-year: 10 %
–
85
Liu et al. [5]
33
3-year: 12.1 %
–
42
Lughezzani et al. [7]
45
–
5-year: 33.2 %
51
Djajadiningrat et al. [9]
19
–
5-year: 17 %
59
Sharma et al. [26]
84
Median: 13.9 months
–
12.1
Zargar-Shoshtari et al. [12]
51
Median: 14.0 months
–
13.3
Lughezzani et al. similarly analyzed risk factors for the presence of pelvic nodal disease in high-risk penile SCC patients [7]. The authors retrospectively evaluated 142 high-risk penile cancer patients treated at their center with 188 groins that were inguinal LN positive. Patients with ≥3 inguinal LN metastases as well as those with a positive inguinal nodal diameter ≥3 cm were at 4.8- and 2.5-fold increased risk, respectively, of having pelvic nodal metastatic spread (p < 0.05). The presence of pelvic LN metastases increased from 0 % in patients with none of the above risk factors to 57 % when all three risk factors were present (p < 0.05).
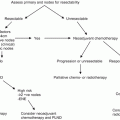
Currently, the National Comprehensive Cancer Network (NCCN)© and European Association of Urology (EAU)© have published guidelines recommending pelvic lymphadenectomy in patients with ≥2 positive inguinal LNs, inguinal ENE, or in the clinical context of high-grade cancer within the inguinal LN pathologic specimen (Fig. 7.1) [15, 16].
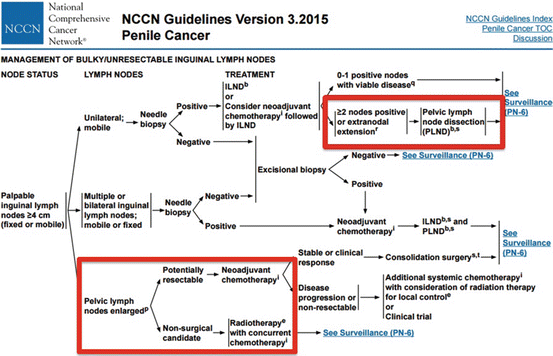

Fig. 7.1
NCCN© penile cancer guidelines for the management of advanced loco-regional nodal disease including pelvic LNs [Reprinted from Clark et al. [36], copyright (2013), with permission from NCCN©]
Surgical Technique
PLND is typically performed through a midline , suprapubic, infraumbilical extraperitoneal incision [17]. Laparoscopic approaches may also be utilized for PLND in order to minimize surgically related morbidity and reduce postoperative complications. The rapid adoption of minimally invasive robotic-assisted surgery can additionally allow for greater accuracy and wrist-motion than traditional laparoscopic instruments due to the magnification and three-dimensional visualization of Da Vinci© technology.
The boundaries of PLND include: superior—common iliac artery and vein bifurcation; lateral—ilioinguinal nerve; and medial—obturator nerve (Fig. 7.2) [9, 12]. During PLND, all nodal tissue is removed from the obturator, internal iliac, and external iliac packets, and any enlarged LNs in the pelvis should also be excised. Meticulous hemostasis during PLND must be achieved to prevent excess venous bleeding and development of a pelvic hematoma [10]. Additionally, ligation or clipping of lymphatic channels is of the utmost importance during PLND to prevent the occurrence of a pelvic lymphocele during the postoperative period.
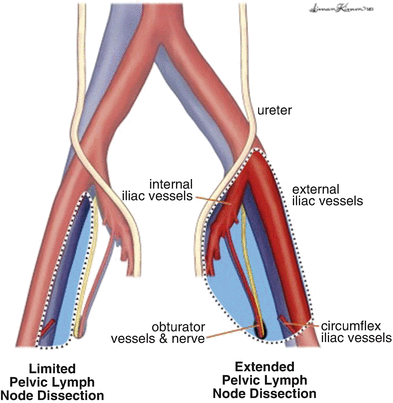

Fig. 7.2
Template for boundaries of dissection during PLND for high-risk penile cancer [Reprinted from Yuh et al. [37], copyright (2014), with permission from Elsevier]
PLND can be done at the same time as inguinal lymph node dissection (ILND) or in a delayed fashion since there is very little evidence to suggest that the timing of PLND in relation to ILND affects clinical outcomes. Currently, PLND is typically performed in a unilateral fashion since no crossover effect has been reported in penile cancer from the inguinal to pelvic LNs, but bilateral PLND may be indicated for bilateral inguinal metastatic disease.
Unilateral Versus Bilateral PLND
Most current recommendations would suggest unilateral PLND in patients with unilateral inguinal LN metastasis. Although bilateral pelvic lymphatic metastatic spread is theoretically possible, it is extremely rare and associated with very poor survival-related outcomes.
One area of controversy is whether the PLND should be performed ipsilaterally or bilaterally in patients with unilateral positive inguinal metastatic disease. Since crossover (right to left or left to right) of inguinal to pelvic nodes has not been well studied, unilateral or bilateral PLND are both feasible approaches and left at the discretion of the surgeon based on case-specific characteristics.
Zargar-Shoshtari et al. retrospectively analyzed 51 men with penile SCC and unilateral inguinal node-positive disease with pelvic LN metastatic disease after ILND and PLND across four international centers of excellence [12]. Thirty-eight men (75 %) in the study population had a unilateral pelvic lymphadenectomy, and 13 (25 %) had bilateral pelvic lymphadenectomy. Patients who underwent unilateral versus bilateral PLND were similar with respect to clinicodemographic criteria, disease-specific characteristics, and utilization of multimodal therapy. Penile cancer patients who underwent bilateral PLND had a statistically significant better estimated median OS compared to those who underwent unilateral PLND (21.7 vs. 13.1, p = 0.05). On multivariate analysis, bilateral PLND [HR: 0.25, (95 % CI: 0.10–0.64)], multiple positive pelvic nodes [HR: 2.12 (95 % CI: 1.02–4.43)], use of neoadjuvant chemotherapy [HR: 0.01, (95 % CI: 0.02–0.44)], and use of adjuvant systemic therapy and/or radiation therapy (compared to surveillance) [HR: 0.16, (95 % CI: 0.06–0.45)] were independently associated with better OS. These findings suggest that men with pelvic node positive penile carcinoma may have improved long-term outcomes from a bilateral PLND.
Zargar-Shostari et al. also evaluated risk factors for bilateral pelvic metastatic lymphatic disease in penile cancer patients with positive inguinal LNs [11]. Sixty-four patients with pelvic node positive disease who underwent bilateral pelvic lymphadenectomy and had positive bilateral inguinal LNs were included for analysis. Bilateral pelvic node positive disease was found in 16 patients (25 %). The detection of four or more positive inguinal LNs had a 95 % sensitivity to predict bilateral pelvic nodal metastasis on final pathology (area under receiver operating characteristic [ROC] curve of 0.76; p < 0.05). Additionally, on multivariate analysis, ≥4 positive inguinal LNs was the only statistically significant predictor of bilateral pelvic LN metastasis (OR: 14.0, 95 % CI: 1.7–115). Variables independently associated with OS included ≥4 positive inguinal LNs, use of adjuvant chemotherapy, inguinal ENE, and a bilateral pelvic lymphadenectomy. These findings were used to establish criteria for bilateral PLND in patients with high-volume inguinal metastatic lymphatic spread (Fig. 7.3). The authors concluded that for patients with bilateral inguinal metastatic lymphatic spread and ≥4 positive inguinal LNs, bilateral PLND should be considered due to a higher risk of bilateral pelvic node positive disease and improved survival.
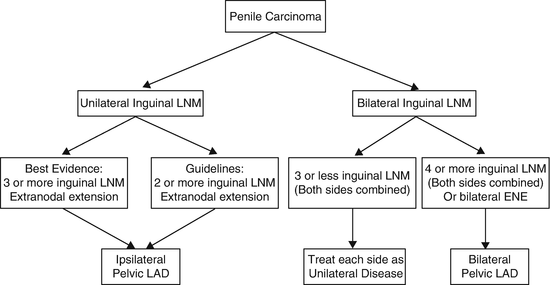

Fig. 7.3
Proposed algorithm to manage high-volume inguinal metastatic nodal disease with unilateral versus bilateral PLND [Reprinted from Zargar-Shoshtari et al. [11], copyright (2015), with permission from Elsevier]
Systemic Therapy for Pelvic Nodal Disease
Although surgery alone may cure a small percentage of high-risk penile cancer patients with micro-metastatic pelvic LN disease (16–20 %), recurrence is very common, and patients may have an overall poor prognosis with a low 5-year OS rate. Patient with pelvic node positive disease, therefore, may benefit from additional systemic chemotherapy due to the high risk of microscopic systemic spread [1, 18].
Adjuvant chemotherapy is recommended by the NCCN© and EAU© for pN2 and pN3 penile SCC based on evidence from well-designed case–control and cohort studies as well as evidence from descriptive and qualitative studies (Table 7.2) [19–22]. Pizzocaro et al. reported that 12 weekly cycles of adjuvant vinblastine, bleomycin, and methotrexate in 25 inguinal node positive patients resulted in a better overall disease-free survival (DFS) rate compared to 38 inguinal node positive patients that did not receive any chemotherapy (84 % vs. 39 %, p < 0.05) [23]. The authors also showed that treatment of 19 inguinal node positive penile cancer patients with three to four cycles of adjuvant docetaxel, cisplatin, and 5-fluorouracil resulted in a 52.6 % DFS rate at a median follow-up of 42 months [15]. Noronha et al. used an alternative regimen of four cycles of adjuvant cisplatin and paclitaxel at 21-day intervals in 19 patients with penile SCC and pN2/N3 disease, and they reported a sustained increase in DFS compared to the control arm of pN2/N3 patients who were just monitored with surveillance (23.1 vs. 2.2 months, p < 0.05) [24].
Houédé et al. analyzed response rates after induction chemotherapy with six cycles of gemcitabine and cisplatin in 25 penile cancer patients with locally advanced, unresectable lymphadenopathy in the pelvis and groin and/or systemic metastatic disease [25]. In this non-randomized, phase II trial, the primary endpoint included the objective response rate with second endpoints of time to progression (TTP), OS, and side effects related to this treatment regimen. Only 6 of 25 patients (24 %) were able to complete the full induction course of chemotherapy. Treatment was stopped in six patients (24 %) because of a severe (grade 3 or 4) toxicity profile with intolerable adverse effects. The overall objective partial or complete response rate was 8 % (n = 2). Thirteen patients (56.5 %) had stable disease after intention-to-treat analysis on follow-up imaging, and eight patients (34.8 %) had progression of their penile SCC. With regards to secondary endpoints, the estimated median TTP was 5.5 months, estimated median OS was 15 months, and estimated 2-year OS in the study population was 39 %.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree