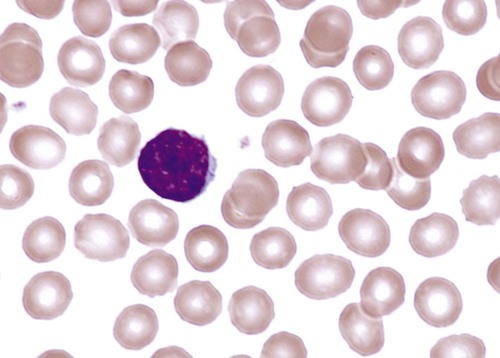
After completion of this chapter, the reader will be able to: 1. Describe the major differences in normal complete blood count values, reticulocytes, and nucleated red blood cells (NRBCs) in preterm newborns, full-term newborns, infants, children, adults, and elderly adults. 2. Explain the cause of physiologic anemia of infancy and the time frame in which it is expected. 3. Describe normal RBC morphology in neonates. 4. Compare the RBC survival in preterm and full-term infants with that in adults. 5. Recognize and list factors affecting sample collection that can have an impact on the interpretation of hematology values in newborns. 6. Compare and contrast the morphology of lymphocytes in children and in adults and indicate reasons for differences. 7. Describe the general association between age and hemoglobin levels in the elderly. 8. Explain the clinical significance of anemia in the elderly. 9. Name the two most common anemias seen in the elderly and their common causes in this age group. 10. List other anemias affecting elderly individuals. 11. Compare the frequency of acute lymphoblastic leukemias and chronic lymphocytic leukemias in children and the elderly. 12. Compare the hemostatic systems of newborns, children, adults, and the elderly, including the risk of bleeding and thrombosis. 13. Name malignancies of hematologic cells that are more common in the elderly than in other age groups. After studying this chapter, the reader should be able to respond to the following case study: 1. Are the results for hemoglobin and hematocrit within the limits expected for a newborn? 2. What is the significance of the elevated MCV, NRBCs, and polychromasia? Dramatic changes occur in the blood and bone marrow of the newborn infant during the first hours and days after birth, and there are rapid fluctuations in the quantities of all hematologic elements. Significant hematologic differences are seen between term and preterm infants and among newborns, infants, young children, and older children. This chapter reviews neonatal hematopoiesis, which is discussed in detail in Chapter 7, as a prerequisite to understanding the changes in pediatric hematologic reference ranges, morphologic features, and age-specific physiology. Hematopoiesis, the formation and development of blood cells from stem cells, begins in the first weeks of embryonic development and proceeds systematically through three phases of development: mesoblastic (yolk sac), hepatic (liver), and myeloid (bone marrow). The first cells produced in the developing embryo are primitive erythroblasts formed in the yolk sac. These cells are particularly interesting because they do not develop into mature erythrocytes. They are erythropoietin insensitive and have the ability to differentiate into other cell lines upon exposure to appropriate growth factors.1–6 By the second month of gestation, hematopoiesis ceases in the yolk sac, and the liver becomes the center for hematopoiesis, reaching its peak activity during the third and fourth gestational months. Leukocytes of each cell type systematically make their appearance. In week 9 of gestation, lymphocytes can be detected in the region of the thymus. They are subsequently found in the spleen and lymph nodes. During the fourth and fifth gestational months, the bone marrow emerges as a major site of blood cell production, and it becomes the primary site by birth (see Chapter 7).1–6 Hematopoietically active bone marrow is referred to as red marrow, as opposed to inactive yellow (fatty) marrow. At the time of birth, the bone marrow is fully active and extremely cellular, with all hematopoietic cell lineages undergoing cellular differentiation and amplification. In addition to the mature cells in fetal blood, there are significant numbers of circulating progenitor cells in cord blood.7,8 In a full-term infant, hepatic hematopoiesis has ceased except in widely scattered small foci that become inactive soon after birth.1–6 Postembryonic extramedullary hematopoiesis is abnormal in a full-term infant. In a premature infant, foci of hematopoiesis are frequently seen in the liver and occasionally observed in the spleen, lymph nodes, or thymus.1–10 Hematologic values obtained from full-term infants generally do not apply to preterm infants, and laboratory values for low-birth-weight preterm infants differ from ranges for extremely-low-birth-weight “micropreemies” (24 to 26 weeks’ gestation).11 A full-term infant is defined as an infant who has completed 37 to 42 weeks of gestation. Infants born before 37 weeks’ gestation are referred to as premature or preterm, whereas infants delivered after 42 weeks are considered postterm.11,12 Infants can be subcategorized further by birth weight as (1) appropriate size for gestational age; (2) small for gestational age, including low-birth-weight infants (2500 g or less); (3) very-low-birth-weight infants (1500 g or less); (4) extremely-low-birth-weight micropreemies (500 g or less); and (5) large for gestational age (more than 4000 g).11 Neonatal hematologic values are affected by the gestational age of the infant, the age in hours after delivery, the presence of illness, and the level of support required. Other important variables to be considered when evaluating laboratory data include site of sampling and technique (capillary versus venous puncture, warm or unwarmed extremity), timing of sampling, and conditions such as the course of labor and the treatment of the umbilical vessels.11,13,14 The presence of fetal hemoglobin (Hb F), bilirubin, and lipids in newborns can also interfere with hematology laboratory testing.5–17 As with all laboratory testing, each laboratory should establish reference intervals based on its instrumentation, methods, and patient population (see Chapter 5). Refer to the inside front cover of this book for red blood cell (RBC) reference ranges. The RBC count increases during the first 24 hours of life, remains at this plateau for about 2 weeks, and then slowly declines. This “polycythemia of the newborn”18 may be explained by in utero hypoxia, which becomes more pronounced as the fetus grows. Hypoxia, the trigger for increased secretion of erythropoietin, stimulates erythropoiesis.19 At birth, the physiologic environment changes, and the fetus makes the transition from its placenta-dependent oxygenation to the increased tissue oxygenation of the lungs. This increased oxygen tension suppresses erythropoietin production, which is followed by a decrease in RBC and hemoglobin production. Studies show that erythropoietin levels before birth are equal to or greater than adult levels with a gradual drop to near zero a few weeks after birth.20–23 This decline corresponds to the physiologic anemia seen at 5 to 8 weeks of life, with the RBCs reaching their lowest count at 7 weeks of age and hemoglobin reaching its lowest concentration at 9 weeks of age.19 Early normoblasts are megaloblastic, hypochromic, and irregularly shaped. During hepatic hematopoiesis, normoblasts are smaller than the megaloblasts of the yolk sac but are still macrocytic. Erythrocytes remains macrocytic from the first 11 weeks of gestation until day 5 of postnatal life (Figure 38-1).* The macrocytic RBC morphology gradually changes to the characteristic normocytic, normochromic morphology. Orthochromic normoblasts frequently are observed in the full-term infant on the first day of life but disappear within postnatal days 3 to 5. These nucleated RBCs (NRBCs) may persist longer than a week in immature infants. The average number of NRBCs ranges from 3 to 10 per 100 white blood cells (WBCs) in a normal full-term infant to 25 NRBCs per 100 WBCs in a premature infant. The presence of NRBCs for more than 5 days suggests hemolysis, hypoxic stress, or acute infection.* The erythrocytes of newborns show additional morphologic differences. The number of biconcave discs relative to stomatocytes is reduced in neonates (43% discs, 40% stomatocytes) compared with adults (78% discs, 18% stomatocytes).27 In addition, increased numbers of pitted cells, echinocytes, spherocytes, and other abnormally shaped erythrocytes are seen in neonates. The number of these “dysmorphic” cells is even higher in premature infants. Zipursky et al found 40% discs, 30% stomatocytes, and 27% additional poikilocytes in premature infants.27 An apparent reticulocytosis exists during gestation, decreasing from 90% reticulocytes at 12 weeks’ gestation, to 15% at 6 months’ gestation, and ultimately to 4% to 6% at birth. Reticulocytosis persists for about 3 days after birth, then declines abruptly to 0.8% reticulocytes on postnatal day 4 to 7. At 2 months, the number of reticulocytes increases slightly, followed by a slight decline from 3 months to 2 years, when adult levels of 0.5% to 1.5% are attained.† The reticulocyte count of premature infants is typically higher than that of term infants; however, the count can vary dramatically depending upon how ill the newborn is. Significant polychromasia seen on a Wright-stained blood film is indicative of postnatal reticulocytosis (Figure 38-2). Hemoglobin synthesis results from an orderly evolution of a series of embryonic, fetal, and adult hemoglobins. At birth Hb F constitutes 70% to 80% of the total hemoglobin.28 Hb F declines from 90% to 95% at 30 weeks’ gestation to approximately 7% at 12 weeks after birth and stabilizes at 3.2 ± 2.1% at 16 to 20 weeks after birth.29 The switch from Hb F to Hb A is genetically controlled and determined by gestational age; it does not appear to be influenced by the age at which birth occurs.30 Chapter 10 provides an in-depth discussion of the ontogeny, structure, and types of hemoglobin. The concentration of hemoglobin fluctuates dramatically in the weeks and months after birth as a result of physiologic changes, and various factors must be considered when analyzing pediatric hematologic values. The site of sampling, gestational age, and time interval between delivery and clamping of the umbilical cord can influence the hemoglobin level in newborn infants.* In addition, there are significant differences between capillary and venous blood hemoglobin levels. Capillary samples in newborns generally have a higher hemoglobin concentration than venous samples, which can be attributed to circulatory factors.† Racial differences must also be considered when evaluating hemoglobin levels in children. African American children have hemoglobin levels averaging 0.5 g/dL lower than those in white children.31 The average hemoglobin for a full-term infant at birth is 16.5 to 21.5 g/dL; levels less than 14 g/dL are considered abnormal.20,21 The average hemoglobin value for a preterm infant who is small for gestational age is 17.1 g/dL, lower than that for a full-term infant; hemoglobin values less than 13.7 g/dL are considered abnormal in preterm infants.21 The hemoglobin concentration of term infants decreases during the first 5 to 8 weeks of life, a condition known as physiologic anemia of infancy. Infants born prematurely also experience a decrease in hemoglobin concentration, which is termed physiologic anemia of prematurity.1,32–34 Along with a decrease in hemoglobin, there is a reduction in the number of RBCs, a decrease in the reticulocyte percentages (Table 38-1), and undetectable levels of erythropoietin associated with the transition from the placenta to the lungs as a source of oxygen. When the hemoglobin concentration decreases to approximately 11 g/dL, erythropoietic activity increases until it reaches its adult levels by 14 years of age.11,18,35–40 Also contributing to the physiologic anemia is the shortened life span of the fetal RBC. Studies of chromium-labeled newborn RBCs estimate a survival time of 60 to 70 days with correction for the elution rate of chromium from newborn cells.1 The life span of RBCs in premature infants is about 35 to 50 days.1 The more immature the infant, the greater the degree of reduction.1,21–23,26,41 The reasons for the shortened life span of the erythrocytes are unclear. This physiologic anemia is not known to be associated with any abnormalities in the infant. Hemodilution related to the increased blood volume that accompanies the rapid weight gain seen in the first few months of life is not thought to play a key role in the anemia.1 TABLE 38-1 Hematologic Values for Very-Low-Birth-Weight Infants During the First 6 Weeks of Life Modified from Obladen M, Diepold K, Maier RF, et al: Venous and arterial hematologic profiles of very low birth weight infants, Pediatrics 106:707-711, 2000. The hemoglobin levels of premature infants are typically 1 g/dL or more below the values of full-term infants. Thereafter, a gradual recovery occurs, which results in values approximating those of normal full-term infants by about 1 year of age.* Very-low-birth-weight infants (less than 1500 g) show a progressive decline in hemoglobin, RBC count, mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC), and have a slower recovery than other preterm and term infants. The average hematocrit (Hct) at birth for full-term infants is 53% (range, 48% to 68%).42 Frequently, newborns with increased hematocrits, especially values greater than 65%, experience hyperviscosity of the blood. This can cause problems in producing a high-quality peripheral blood film. Very-low-birth-weight preterm infants are frequently anemic at birth (see Table 38-1). Many require transfusions or erythropoietin injections or both. The RBC indices and RBC distribution width (RDW) provide a means for assessing and defining anemia (see Chapter 18). The erythrocytes of newborn infants are markedly macrocytic at birth. The average MCV for full-term infants is 110 ± 15 fL; however, a sharp decrease occurs during the first 24 hours of life. The MCV continues to decrease to 90 ± 12 fL in 3 to 4 months.18,37 The more premature the infant, the higher the MCV. A newborn with an MCV of less than 94 fL should be evaluated for α-thalassemia or iron deficiency.1,43 Nutritional deficiencies in infants and children can result in iron deficiency anemia and, rarely, in megaloblastic anemia (see Chapter 20), particularly in low-birth-weight and premature infants. These anemias are associated with abnormal psychomotor development; however, they can easily be treated with dietary fortification.44–48 Iron deficiency anemia is the most common pediatric hematologic disorder and the most frequent cause of anemia in childood.49 The occurrence of iron deficiency anemia in infants has decreased in the United States due to iron fortification of infant formula and increased rates of breastfeeding.50 However, the prevalence is still 2% in toddlers 1 to 2 years of age and 3% in children 3 to 5 years of age51 and is related to early introduction and excessive intake of whole cow’s milk.44,52 Refer to Chapter 19 for an in-depth discussion of iron deficiency anemia. The differential diagnosis of anemia in infants and children relies on a variety of ancillary tests. The reference ranges for a number of these tests differ from those for adults. Haptoglobin levels are so low as to be undetectable in neonates, which makes it unreliable as a marker of infant hemolysis.53 Transferrin levels are also lower in neonates, increasing rapidly after birth and reaching adult values at 6 months.53 Both serum ferritin and serum iron are high at birth, rise during the first month, drop to their lowest level between 6 months and 4 years of age, and remain low throughout childhood.54–56 Consideration of these differences is important when interpreting hematology laboratory results for infants and children. Fluctuations in the number of WBCs are common at all ages but are greatest in infants (Table 38-2). Leukocytosis is typical at birth for full-term and preterm infants, with a wide range of normal.57 There is an excess of segmented neutrophils and bands, and an occasional metamyelocyte, with no evidence of disease. The neutrophilic leukocyte count rises within the first 12 hours following birth, decreases between 1 month and 1 year, then gradually increases to stabilize at 4.4 × 109/L at about 4 years of age.57 TABLE 38-2 *Numbers of leukocytes are × 109/L (or thousands per microliter), ranges are estimates of 95% confidence limits, and percentages refer to differential counts. †Neutrophils include band cells at all ages and a small number of metamyelocytes and myelocytes in the first few days of life. ‡Dashes indicate insufficient data for a reliable estimate. From Cairo MS, Bracho F: White blood cells. In Rudolph CD, Rudolph AM, Hostetter MK, et al, editors: Rudolph’s pediatrics, ed 21, New York, 2003, McGraw-Hill, p 1548. Refer to Table 38-2 and the inside front cover for the leukocyte reference ranges for full-term infants. Term and premature infants show a greater absolute neutrophil count than that found in older children, who characteristically maintain a predominance of lymphocytes. Band forms are also higher for the first 3 to 4 days after birth (Table 38-3). Newborn females have neutrophil counts averaging 2000 cells/mcL higher than those of males; neonates whose mothers have undergone labor have higher counts than neonates delivered by cesarean section with no preceding maternal labor.58,59 There is some evidence that absolute neutrophil counts are lower in healthy black children than in white children.60 TABLE 38-3 Neutrophil and Band Counts for Newborns during the First 2 Days of Life* *Normal values were obtained from the assessment of 3100 separate white blood cell counts obtained from 965 infants; 513 counts were from infants considered to be completely normal at the time the count was obtained and for the preceding and subsequent 48 hours. There was no difference in the normal ranges when values were stratified by infant birth weight or gestational age. Modified from Luchtman-Jones L, Schwartz AL, Wilson DB: Hematologic problems in the fetus and neonate. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s neonatal-perinatal medicine, ed 8, Philadelphia, 2006, Mosby Elsevier, p 1310. At birth, preterm infants exhibit a left shift, with promyelocytes and myelocytes frequently observed. The trend to lymphocyte predominance occurs later than in full-term infants. The neutrophil counts in premature infants are similar to or slightly lower than the neutrophil counts in full-term infants during the first 5 days of life; however, the count gradually declines to 2.5 × 109/L (1.1 to 6.0 × 109/L) at 4 weeks.61 There is no significant difference in the neutrophil count of infants by birth weight or gestational age; however, very-low-birth-weight infants have a significantly lower limit (1 × 109/L) compared with larger infants.61–63 Neutropenia is defined as a reduction in the number of circulating neutrophils to less than 1.5 × 109/L. Neutropenia accompanied by bands and metamyelocytes is often associated with infection, particularly in preterm neonates. Neutropenia represents a decrease in neutrophil production or an increase in consumption.64 Lymphocytes constitute about 30% of the leukocytes at birth and increase to 60% at 4 to 6 months. They decrease to 50% by 4 years, to 40% by 6 years, and to 30% by 8 years.13,14,20,24 Benign immature B cells (hematogones), although predominantly found in the bone marrow, can sometimes be seen in the peripheral blood of newborns. These lymphocytes are primarily mid-stage B cells66,67 and are frequently referred to as “baby” or “kiddie” lymphocytes. They vary in size from 10 to 20 µm, have scant cytoplasm and condensed but homogeneous nuclear chromatin, and may have small, indistinct nucleoli (Figures 38-3 and 38-4).68–70 Although these lymphocytes may be similar in appearance to the malignant cells seen in childhood acute lymphoblastic leukemia (ALL), these benign cells lack the asynchronous or aberrant antigen expression seen in ALL and thus can be differentiated from the lymphocytes of infant ALL by immunophenotyping (see Chapter 33).71,72 Sepsis in neonates is a common cause of morbidity, particularly in premature and low-birth-weight infants.73–77 Defective B-cell response against polysaccharide agents as well as abnormal cytokine release by neutrophils and monocytes has been implicated.78–81 Because of the transient neutrophilia that occurs during the first 24 hours after birth, followed by a rapid decline, the neutrophil count is not a satisfactory index of infection in the newborn.82 Newborns with bacterial infections frequently have normal or below normal neutrophil counts with a shift to the left. Thus, many practitioners depend upon the band count and its derived immature-to-total neutrophil ratio as an indicator of sepsis in neonates,83 although CD64, C-reactive protein, and procalcitonin levels have been suggested as more sensitive markers of sepsis in infants and children.83,84 The platelet count usually ranges from 150 to 400 × 109/L for full-term and preterm infants, comparable to adult values (Table 38-4).85,86 Platelet counts generally increase in both term and preterm infants in the first few months of life, as evidenced by increased mean platelet volume the first month of life. Thrombocytopenia of fewer than 100 × 109 platelets/L may be seen in high-risk infants with sepsis or respiratory distress and neonates with trisomy syndromes, and investigation should be undertaken for underlying pathology.87,88 Platelets of a newborn infant show great variation in size and shape. Neonatal thrombocytopenia is discussed in Chapter 43. TABLE 38-4 Normal Platelet Counts for Full-Term and Preterm Infants Adapted from Oski FA, Naiman JL: Normal blood values in the newborn period. In Hematologic problems in the newborn, Philadelphia, 1982, WB Saunders. The physiology of the hemostatic system in infants and children is different from that in adults (see Chapter 40). The vitamin K–dependent coagulation factors (factors II, VII, IX, and X) are at about 30% of adult values at birth; they reach adult values after 2 to 6 months, although the mean values remain lower in children than in adults. Levels of factor XI, factor XII, prekallikrein, and high-molecular-weight kininogen are between 35% and 55% of adult values at birth, reaching adult values after 4 to 6 months. In contrast, the levels of fibrinogen, factor VIII, and von Willebrand factor are similar to adult values throughout childhood.89,90 Factor V decreases during childhood, with lower levels during the teen years as compared with adults. The physiologic anticoagulants and inhibitors of coagulation—protein C, protein S, antithrombin, and a disintegrin-like and metalloprotease domain with thrombospondin type 1 motifs 13 (ADAMTS 13)—are reduced to about 30% to 40% at birth. Antithrombin reaches adult values by 3 months, whereas protein C does not normalize until after 6 months.89 In the fibrinolytic system, levels of plasminogen and α2-antiplasmin are similar to adult levels at birth, whereas levels of tissue plasminogen activator are low and levels of plasminogen activator inhibitor (PAI) are increased (Table 38-5).90 The hemostatic components are not only changing in concentration over the first few weeks to months of life, but their values are also dependent upon the gestational age of the child, and premature infants have different values at birth than term infants (Table 38-6).
Pediatric and Geriatric Hematology
Case Study
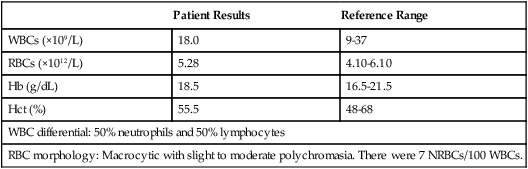
Patient Results
Reference Range
WBCs (×109/L)
18.0
9-37
RBCs (×1012/L)
5.28
4.10-6.10
Hb (g/dL)
18.5
16.5-21.5
Hct (%)
55.5
48-68
WBC differential: 50% neutrophils and 50% lymphocytes
RBC morphology: Macrocytic with slight to moderate polychromasia. There were 7 NRBCs/100 WBCs.
Pediatric Hematology
Prenatal Hematopoiesis
Hematopoiesis of the Newborn
Gestational Age and Birth Weight
Red Blood Cell Values at Birth
Red Blood Cell Count
Erythrocyte Morphology of the Neonate
Reticulocyte Count
Hemoglobin
Full-Term Infants
Physiologic Anemia of the Neonate
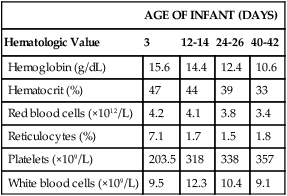
AGE OF INFANT (DAYS)
Hematologic Value
3
12-14
24-26
40-42
Hemoglobin (g/dL)
15.6
14.4
12.4
10.6
Hematocrit (%)
47
44
39
33
Red blood cells (×1012/L)
4.2
4.1
3.8
3.4
Reticulocytes (%)
7.1
1.7
1.5
1.8
Platelets (×109/L)
203.5
318
338
357
White blood cells (×109/L)
9.5
12.3
10.4
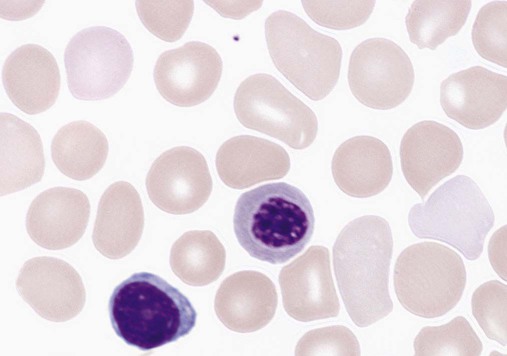
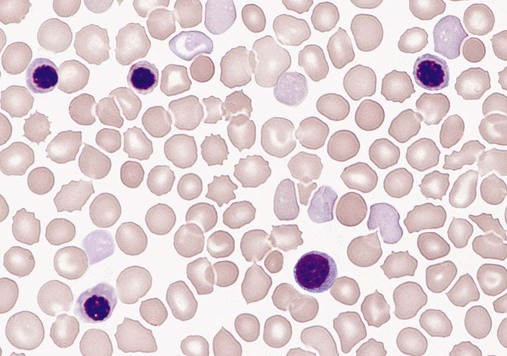
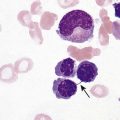
9.1
Hematocrit
Red Blood Cell Indices
Mean Cell Volume
Anemia in Infants and Children
Iron Deficiency Anemia
Ancillary Tests for Anemia in Infants and Children
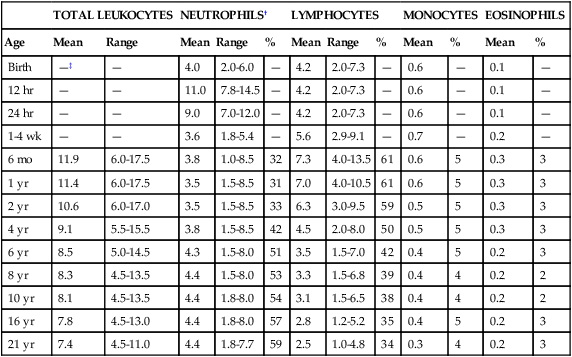
White Blood Cell Values in the Newborn
TOTAL LEUKOCYTES
NEUTROPHILS†
LYMPHOCYTES
MONOCYTES
EOSINOPHILS
Age
Mean
Range
Mean
Range
%
Mean
Range
%
Mean
%
Mean
%
Birth
—‡
—
4.0
2.0-6.0
—
4.2
2.0-7.3
—
0.6
—
0.1
—
12 hr
—
—
11.0
7.8-14.5
—
4.2
2.0-7.3
—
0.6
—
0.1
—
24 hr
—
—
9.0
7.0-12.0
—
4.2
2.0-7.3
—
0.6
—
0.1
—
1-4 wk
—
—
3.6
1.8-5.4
—
5.6
2.9-9.1
—
0.7
—
0.2
—
6 mo
11.9
6.0-17.5
3.8
1.0-8.5
32
7.3
4.0-13.5
61
0.6
5
0.3
3
1 yr
11.4
6.0-17.5
3.5
1.5-8.5
31
7.0
4.0-10.5
61
0.6
5
0.3
3
2 yr
10.6
6.0-17.0
3.5
1.5-8.5
33
6.3
3.0-9.5
59
0.5
5
0.3
3
4 yr
9.1
5.5-15.5
3.8
1.5-8.5
42
4.5
2.0-8.0
50
0.5
5
0.3
3
6 yr
8.5
5.0-14.5
4.3
1.5-8.0
51
3.5
1.5-7.0
42
0.4
5
0.2
3
8 yr
8.3
4.5-13.5
4.4
1.5-8.0
53
3.3
1.5-6.8
39
0.4
4
0.2
2
10 yr
8.1
4.5-13.5
4.4
1.8-8.0
54
3.1
1.5-6.5
38
0.4
4
0.2
2
16 yr
7.8
4.5-13.0
4.4
1.8-8.0
57
2.8
1.2-5.2
35
0.4
5
0.2
3
21 yr
7.4
4.5-11.0
4.4
1.8-7.7
59
2.5
1.0-4.8
34
0.3
4
0.2
3
Neutrophilic Leukocytes
Age
Absolute Neutrophil Count (× 109/L)
Absolute Band Count (× 109/L)
Birth
3.5-6.0
1.3
12 hr
8.0-15.0
1.3
24 hr
7.0-13.0
1.3
36 hr
5.0-9.0
0.7
48 hr
3.5-5.2
0.7
Premature Infants
Neutropenia
Lymphocytes
Monocytes
Neonatal Hematologic Response to Infection
Platelet Values in the Newborn
Age
Platelet Count (× 109/L; mean ± 1 SD)
Preterm infants, 27-31 wk
275.0 ± 60.0
Preterm infants, 32-36 wk
290.0 ± 70.0
Term infants
310.0 ± 68.0
Normal child/adult
300.0 ± 50.0
Neonatal Hemostasis
Pediatric and Geriatric Hematology