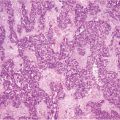
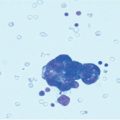
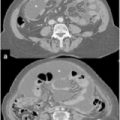
Fig. 13.1
Peritoneal carcinomatosis from ovarian cancer: involvement of lesser omentum and hepatoduodenal ligament
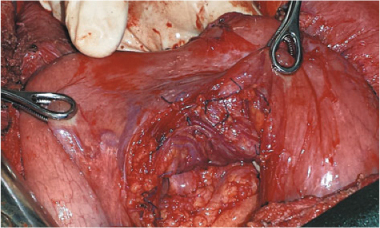
Fig. 13.2
Lesser omentectomy and stripping of the omental bursa floor
Another challenging problem, especially in patients with CRC, concerns coexisting PC with liver metastases. A meta-analysis conducted by Dutch investigators showed a trend toward lower OS after curative resection plus HIPEC in patients with CRC metastases in the liver, as well as in the peritoneum, than in patients with peritoneal metastases alone after the same treatment [48]. Although this approach can be used to treat PC and liver metastases during the same procedure in selected patients, only patients who have limited peritoneal spread (PCI < 12) and a limited extent of liver disease (liver metastases < 3) really benefit from this approach, with a median survival of 40 months [49].
13.5 Decision Making
In his “Presidential Address to the Society of Surgical Oncology,” Blake Cady stated that tumor biology is the king, patient selection is the queen, and technical procedure the prince, of the kingdom. Only rarely does the prince usurp the kingdom [50]. These considerations perfectly illustrate the problem being addressing. Patient selection criteria must maintain the balance between opposing forces. On the one side is a frequently unfavorable and undoubtedly aggressive tumor biology; on the other side is a surgeon convinced that technical efforts will snatch the patient away from an ineluctable destiny. Ideal selection criteria should indicate patients for whom surgery is most likely to have a successful outcome and therefore in whom technical, organizational, and economic efforts, necessitated by these procedures, should be invested.
Research over the past few years, limited to PC from CRC, has developed a staging classification intended chiefly for preoperative use [Peritoneal Surface Disease Severity Score (PSDSS)], which analyzes separately the entity of patient symptoms, the extent of peritoneal disease (PCI), and the histopathologic tumor features (including lymphatic spread), yielding a specific score and then subdivided into four stages (Fig. 13.3) [32]. The American Society of Peritoneal Surface Malignancies conducted a retrospective review of 609 patients who underwent CRS plus HIPEC after PSDSS staging. Their findings show that the PSDSS, assessed before surgery, can define a population with a statistically high or notably low likelihood of long-term survival (Fig. 13.4) [51].
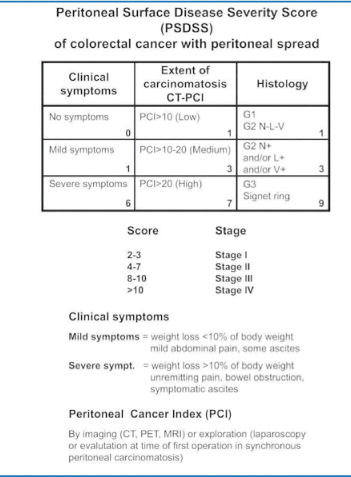
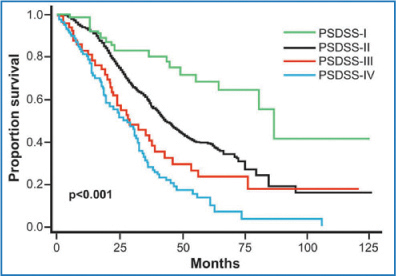

Fig. 13.3
Peritoneal Surface Disease Severity Score (PSDSS). (Reproduced from [52], with permission)

Fig. 13.4
Long-term survival according to the Peritoneal Surface Disease Severity Score (PSDSS). (Reproduced from [52], with permission)
Regardless, whatever the pros and cons of one scoring system or another, the intellectual rationale for selecting patients as candidates for CRS plus HIPEC procedures never concerns the surgeon alone. The decision inevitably requires a multidisciplinary contribution involving many specialists and includes the patients themselves so as to offer an individualized treatment option. Especially important is the need to alert the medical community (medical oncologists, the message is for you) that PSM is not by definition incurable. Quite the contrary: CRS plus HIPEC offers many of these patients a real chance of cure. Only keeping these management options firmly in mind will increase the number of patients referred to expert centers for treatment, help identify patients with PSM at earlier stages and thus simplify patient selection.
References
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
Votanopoulos KI, Swords DS, Swett KR et al (2013) Obesity and peritoneal surface disease: outcomes after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for appendiceal and colon primary tumors. Ann Surg Oncol 20:3899–904CrossRefPubMedCentralPubMed
11.
12.
Bijelic L, Yan TD, Sugarbaker PH (2008) Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J Surg Oncol 98:295–299CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree