Fig. 4.1
p53-positive staining of small aggregates of neoplastic cells in the fimbria (IHC-P, × 50)
4.2.1 Pathology
What the pathologist receives after a CRS procedure is a complex puzzle of specimens of different origin. One of them is the peritoneal layer removed because of gross disease. Peritoneal specimens are sampled from the subdiaphragmatic region, left and right abdominal wall, pelvic and prevesical region, and Morrison’s pouch. Specimens have different shapes, being quadrangular, triangular, or very irregular with several projections and infoldings. The affected peritoneum usually shows punctiform hemorrhages and is slightly thickened by edema or fibrosis of the subperitoneal connective tissue.
Whitish or pale gray nodules can be observed on the serosal surface, measuring from very tiny spots up to several centimeters in maximum dimension (Fig. 4.2a, b); on the cut section, they are seen to extend deep into the subperitoneal connective tissue or fat (Fig. 4.2c). Macroscopic foci of necrosis are often present in the larger nodules of several centimeters in diameter. Microscopically, nests or sheets of cells growing in a solid pattern, with pleomorphic nuclei and occasionally conspicuous nucleoli are observed. Alternatively, tumor cells may form a papillary growth or microcysts.
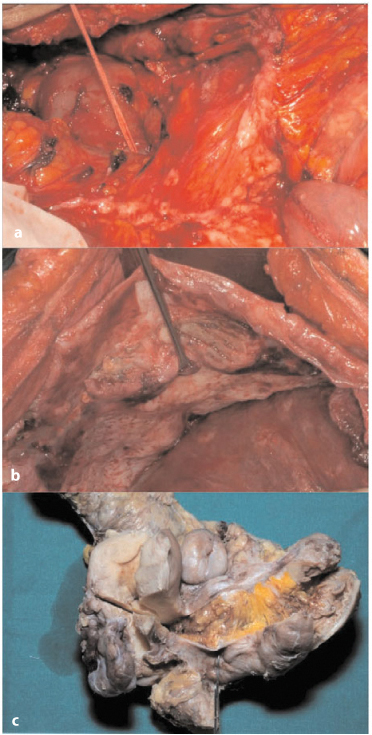

Fig. 4.2a–c
a Small superficial nodules of the lesser omentum. b Plaque-like neoplastic growth in the subdiaphragmatic region. c Superficial deposit and deep infiltrating neoplastic tissue in the pouch of Douglas
In the smaller peritoneal tumor deposits, only tiny papillae or cell aggregates, surrounded by desmoplastic fibrous tissue, are seen. Nodules can have an outgrowth on the peritoneal surface (Fig. 4.3a) or appear in the thickness of the peritoneal sample growing toward the subperitoneal fat tissue (Fig. 4.3b) Often they are multiple and very small (Fig. 4.4a) or are an incidental finding in an apparently noninvolved area of the peritoneum (Fig. 4.4b).
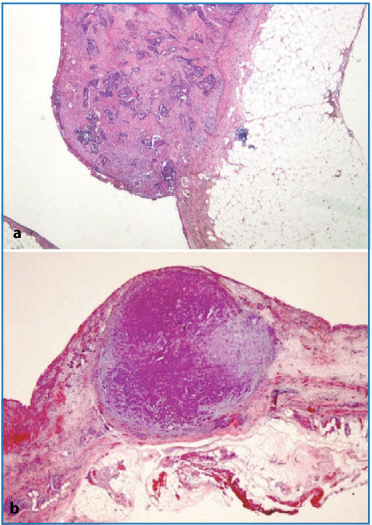
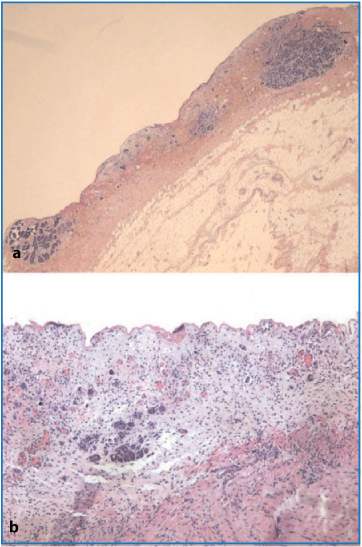

Fig. 4.3a,b
a Exophytic nodule on the peritoneal surface (H&E, × 12.5). b Nodule growing in the thickness of the peritoneal subserosal tissue (periodic acid- Schiff-diastase × 12.5)

Fig. 4.4a,b
a Multiple small neoplastic intraperitoneal nodules (H&E, × 12.5). b Microfocus of neoplastic cells infiltrating the subperitoneal tissue, with no macroscopic alteration on the peritoneal surface (H&E, × 50)
In a minority of cases with very good response to chemotherapy, histology demonstrates subperitoneal foci of desmoplastic tissue with psammoma bodies, with no evidence, or a barely visible aggregate, of malignant cells after a thorough search in multiple samples. In these cases, the psammoma bodies are the distinctive hallmark of the previous solid or papillary neoplastic growth in the subperitoneal tissue (Fig. 4.5).
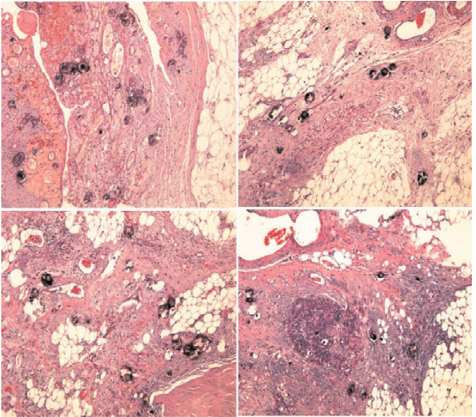

Fig. 4.5
Psammoma bodies inside fibrous desmoplastic tissue, with no or very minimal neoplastic residue (lower right) (H&E, × 50)
The omentum (Fig. 4.6a) is always part of surgical resection, and its macroscopic aspect can vary from that of an omental cake to an intermediate multiand macronodular involvement, to that of very small nodules or irregular thickening in the omental tissue. Omental cake and multimacronodular involvement are associated with massive neoplastic microscopic infiltrates, resulting in diffuse replacement of the omental adipose tissue by whitish, solid, homogenously granular neoplastic tissue. Problems can arise in the macroscopically “normal” omentum, in which an exhaustive sampling must be performed to avoid a misleading diagnosis of absence of neoplastic tissue.
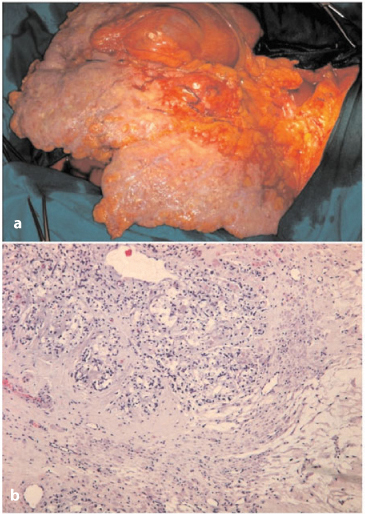

Fig. 4.6a,b
a Massive involvement of the omentum (omental cake). b Signet-ring-cell carcinoma infiltrating fibrous cord of round ligament (H&E, × 50)
The round ligament (Fig. 4.6b) is also usually sampled and may present superficial nodules or an even surface; however, often on serial sectioning, small nodules can be observed in the subperitoneal fat or in fibrous cord residue of the umbilical vessel. Microscopically, solid or papillary tumor deposits with infiltrating edges are seen within adipose tissue or the fibrous cord.
Several specimens labelled as implant(s) are also received. These can be very small fragments, of irregular shape and with variable texture and color; or they may measure a few centimeters in maximum dimension. They are sampled from the visceral peritoneum (small-bowel tracts, gastric wall, left or right colic angles, large-bowel tracts, liver capsule, lesser omentum, Morrison’s pouch). Histologically, all ranges of tumors involvement are possible-from massive infiltration to absence of neoplastic tissue. Small- and large-bowel implants often show tumoral deposits in the subserosa or in the muscle layer, whereas the submucosa and mucosa are frequently involved. Liver-capsule implants are usually exophytic, with no involvement of the underlying liver parenchyma, as the liver capsule can behave as a barrier to liver tissue.
Segments of small and large bowel are often sent for pathological examination and may present multiple micronodules on the serosa or in the subserosa (Fig. 4.7a, b). These can be very small and easily confused with nonneoplastic fibrous thickening of the subserosal fat; consequently, for this reason, any suspicious whitish gray lesion should be sampled. In the more advanced cases, infiltrating lesions appear as a visible ulcerated thickening of the bowel wall, with deeper growths involving the muscular layer (Fig. 4.7c) and possibly submucosa and mucosa (Fig. 4.8a–c). Perivisceral lymph nodes may be enlarged as the result of metastatic growth. They are not necessarily adjacent or close to neoplastic lesions. The finding of lymph node “metastasis” in these specimens is not because of direct invasion, which prompts the idea that after neoplastic nodule establishment in the pericolic adipose tissue, there is diffusion via lymphatic vessels that mimics diffusion of the advanced primary in the intestinal mucosa. The fact that neoplastic cells shed from pericolic nodules and travel in the lymphatic network along the viscera could be a possible cause of recurrence. In a study conducted at our institution [13] on a cohort of patients who underwent CRS plus HIPEC for primary advanced or recurrent OC, we encountered two interesting findings: First, the depth of colorectal wall invasion is an independent, unfavorable prognostic factor for overall survival (OS) equal to the amount of residual disease; in fact this effect can be explained by multiple regression analysis in which the depth of invasion correlates with more extensive peritoneal spread and overall lymph node metastasis. Two other studies show conclusions that agree, at least partly, with our finding [14, 15]. Second, we found a 42.3 % metastatic spread to mesenteric lymph nodes, in keeping with another study [16]. Other authors report figures that differ significantly from ours [15, 17] and correlate it to certain pathologic variables, such as depth of colorectal-wall invasion [15], spread to retroperitoneal lymph nodes [17], or both [18], or the amount of the large bowel resected [14]. In our experience, whenever metastatic peritoneal spread invades the colorectal wall, one can reasonably expect mesenteric lymph node metastasis equal to or more frequent than that found in the pelvic and interaortocaval (locoregional) stations. Regarding the site of lymph nodes involved, we found that mesorectal lymph node metastasis was present in 41.6 % of patients with mesorectal resection, justifying the preferred surgical approach of an almost total mesorectal resection in patients with deep infiltration of the peritoneal pouch and intraperitoneal rectum. Mesenteric lymph node and concurrent locoregional metastases appear to worsen the prognosis, and unsystematic removal of these lymph nodes could result in undiagnosed and residual metastatic disease, a fact that presumably could change this observation, with a less favorable survival for patients with metastatic spread to the mesenteric lymph node [13].
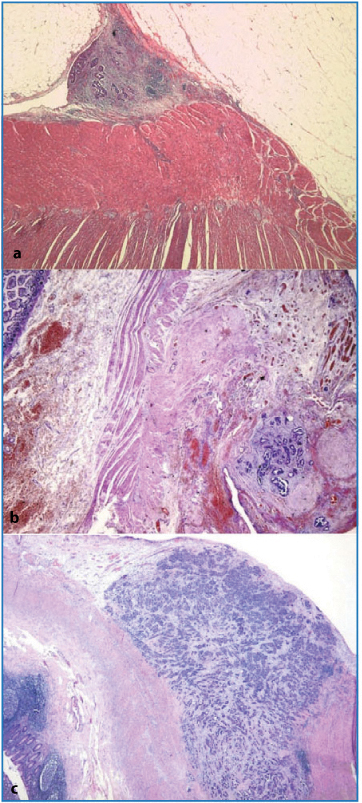
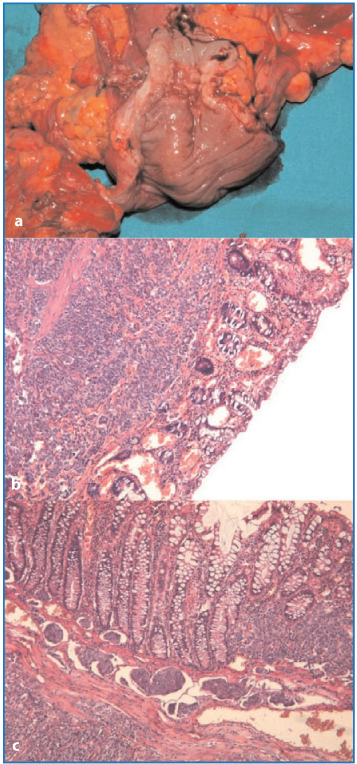

Fig. 4.7a–c
Superficial (a) and subserosal (b) nodules (ab extrinseco infiltrative growth) (H&E, × 12.5]. c Neoplastic tissue infiltrating up to the muscular layer of the gastrointestinal tract (H&E, × 12.5)

Fig. 4.8a–c
a Macroscopic evidence of colonic mucosal infiltration. b Neoplastic involvement of submucosa and deep mucosa (H&E, × 50). c Neoplastic growth in the chorion of the mucosa and mucosal-submucosal lymphatic infiltration (H&E, × 50)
The pouch of Douglas is very frequently involved and shows gross exophytic lesions originating from subperitoneal fat (Fig. 4.9a, b); alternatively lesions are more subtle, and only a thickening of the submesothelial tissue is observed, which corresponds microscopically to small foci of papillary elements or solid paucicellular nests surrounded by desmoplastic fibrous tissue. From the bottom of the pouch of Douglas, lesions can expand to the colic wall or toward the uterus and/or broad ligament. An appropriate sampling of these areas is mandatory when the pathology is not clearly obvious.
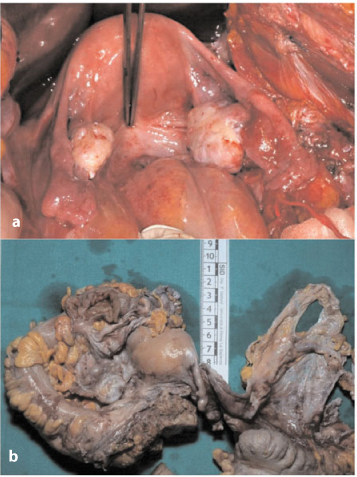

Fig. 4.9a, b
a Nodules and plaque-like growth in the pouch of Douglas. b Nodules in the pouch of Douglas (left) and pelvic parietal peritoneum (right)
The spleen and adipose tissue of the splenic hilus are also typical sites of involvement (see Chap. 9, Fig. 9.14). Usually, neoplastic nodules are contained in the hilar adipose tissue, and, if large, may be visible on the peritoneal surface. Also, the splenic fibrous capsule may present frank nodules or a microscopic nest of neoplastic cells. Very rarely, we have observed neoplastic infiltration of the splenic parenchyma, which in all cases was near the parahilar region, probably where the capsule is thinner and exceptionally we found involvement of an accessory spleen (Fig. 4.10).
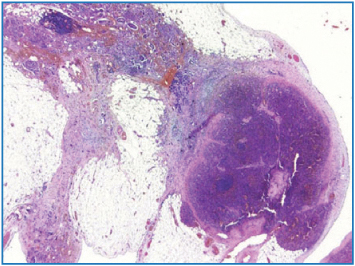

Fig. 4.10
Neoplastic infiltration of accessory spleen (H&E, × 12.5
Regional lymph nodes for the ovary are located in the pelvis and along the iliac artery. They have a typical elongated shape and usually do not harbor metastasis after systemic chemotherapy. A frequent histological finding is that of fibrous and hyaline tissue—often calcified—in the subcapsular sinuses, which makes the lymph node hard and suspicious for metastasis.
If the uterus and adnexa are part of the surgical resection, the most frequent observation is that of bilateral pathological ovaries, which are variably increased in diameter, with frequent adhesion to the uterus and/or to the large bowel. The surface of the ovary is rough due to the outgrowth of the tumor beyond it. In the majority of cases, lesions are solid, with a variable portion of cystic aspect. In the more florid cases, paratubaric tissues and broad ligaments can harbor the neoplastic tissue. The corpus uteri may show serosal nodules that may or may not extend deep beyond the subserosal tissue to reach the myometrium directly. The latter can be involved lymphatically, as well.
4.3 Peritoneal Carcinomatosis from Colorectal and Appendiceal Tumors
Pseudomyxoma peritonei (PMP), also known as mucinous or gelatinous ascites, is a rare condition, with an incidence of ∼ 1/1,000,000 per year [19]. PMP is a clinical term and, therefore, should not be used as a pathological diagnosis. It corresponds to the intraoperative clinical finding of mucus or gelatinous fluid in a localized or generalized form occupying the pelvic or abdominal cavity with involvement of the omentum. PMP is most probably the result of an appendiceal (Fig. 4.11a) or, more rarely, another type of mucus-secreting tumor in the area of the GI tract other than the appendix. Most cases of PMP originate from ruptured low-grade appendiceal tumors, as shown by molecular genetic and immunohistochemical studies [20, 21]. In many cases, the appendiceal tumor is not easily recognized grossly, and therefore, the entire appendix must be serially sectioned and submitted in its entirety for microscopic examination in order to determine the primary site (Fig. 4.11b).
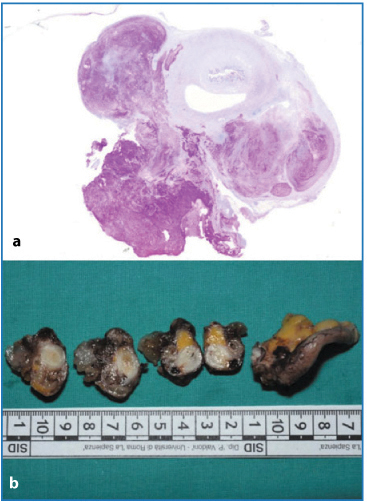

Fig. 4.11a,b
a Huge mucous deposit on appendix serosal surface (periodic acid-Shiff). b Serial sampling of appendix showing neoplastic alteration at different levels
A proposed classification of mucinous tumors involving the peritoneal cavity recognizes two main categories based solely on pathologic features: disseminated peritoneal adenomucinosis (DPAM), and peritoneal mucinous carcinomatosis (PMCA), the prognostic utility of which has been confirmed by followup data with long-term survival for DPAM and very poor prognosis for PMCA patients [22]. Microscopic criteria defining DPAM include disease characterized by histologically bland to low-grade adenomatous mucinous epithelium associated with abundant extracellular mucin and fibrosis, often with an identifiable appendiceal mucinous adenoma or a mucocele. Only rarely are lesions identified within lymph nodes or seen invading the parenchyma of abdominal or pelvic organs. In patients with PMCA, the disease is characterized by peritoneal lesions displaying cytologic and architectural features of mucinous carcinoma associated with extracellular mucin, often with an identifiable invasive mucinous adenocarcinoma of the GI tract. Not infrequently in these cases, parenchymal and lymph node metastases are associated.
A third category has been described and named “peritoneal mucinous carcinomatosis with intermediate or discordant features (PMCA I or D).” This category involves peritoneal lesions that combine DPAM and PMCA-I or markedly atypical appendiceal adenomas associated with peritoneal lesions similar to PMCA-D. However, resection specimens of multivisceral CRS show that in PMP, both low- and high-grade features are seen concurrently at lightmicroscopy examination. Additionally, several authors reported that despite the low-grade histological features and the long-term prognosis of DPAM, multiple disease relapses always occur, leading eventually to death. A claim for using the term mucinous carcinoma peritonei, either low or high grade, for all cases of PMP was recently made by Bradley et al. [23], who showed that the nomenclature of the condition is still hindered by controversy and confusion.
In our experience, true cases of DPAM show multiple nodular formations— soft, bubble-like, and translucent-covering wide areas of the peritoneum: subdiaphragm right and left (Fig. 4.12a), gastric, parietal right and left, spleen surface, omentum, prevesical peritoneum, and pouch of Douglas areas. At microscopic observation, we found mucus deposits covered by a very thin, fibrous, and transparent capsule harboring neutrophils and plasma cells with a sheet of adenomatous epithelium (Fig. 4.12b). The mesothelium appears hyperplastic, with mesothelial cells showing variable size and shape and being polygonal or elongated. These cells stain positively both with cytokeratin (CK) and vimentin, featuring the immunoprofile of the mesothelium. In no cases have we found infiltration of subserosal tissues.
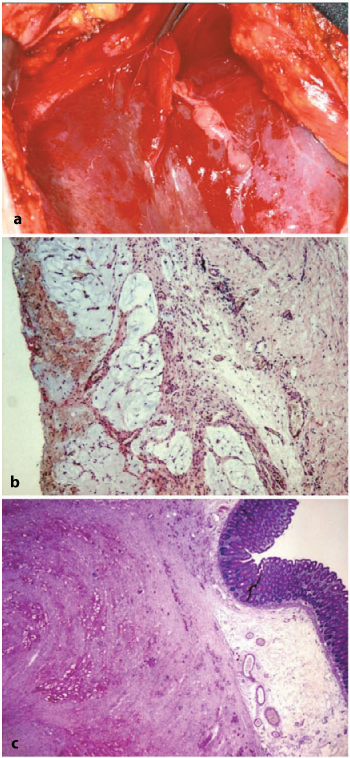

Fig. 4.12a–c
a Soft, bubble-like lesion on the liver-subdiaphragmatic surface typical of disseminated adenomucinosis. b Mucous deposits with subtle fibrous lobulation and inflammatory cells (H&E, × 50). c Signet-ring cells and mucous infiltration of gastrointestinal wall (muscle layer and submucosa) (periodic acid- Schiff-diastase, × 12.5)
PMC shows a more infiltrating tendency, and in this setting, we observed an overlap of pathological involvement of the peritoneal cavity either from a known primary tumor from a specific site in the GI tract (small and large bowel, stomach) or from a tumor putatively originating from appendix.
Small, multiple nodular deposits may be observed that remain superficial or subserosal; deeper lesions invade ab extrinseco toward the internal layers, reaching the mucosa, with concomitant and progressively severe narrowing of the lumen (Fig. 4.12c). Omentum is a preferred site of neoplastic deposits either from advanced primary tumor or in metachronous PC if not previously removed. There is usually involvement of the parietal peritoneum and the pouch of Douglas, where neoplastic growth can be exophytic or invade the underlying adipose tissue. The bladder and genital organs (uterus and ovaries, less frequently prostate) are usually part of the pathological picture of PC, taking the form of direct infiltration from a sigmoid/rectal large-bowel tumor or as seeding of neoplastic cells from tumors of more proximal tracts (i.e., Krukenberg tumor of the ovaries).
Ovarian mucinous adenocarcinomas associated with PC from GI cancer may be erroneously considered as primary ovarian neoplasms, particularly if they are discovered during the first operative procedure, but molecular analysis demonstrates identical KRAS mutations in the large bowel/appendiceal tumor and synchronous ovarian tumors. Ovarian metastases result from neoplastic mucinous cells being deposited on the ovarian surface or having penetrated the ovarian stroma.
4.4 Peritoneal Carcinomatosis from Gastric Cancer (GC)
In the Far East, the most common pattern of disease failure after curative resection of GC is PC [24]. However, little is known about the actual incidence of PC in the setting of GC. A study based on the Eindhoven Cancer Registry population of 2,029 patients diagnosed with GC in The Netherlands found a 39 % rate of metastatic disease, of which 35 % developed PC in the metastatic setting; in 24 %, the peritoneum was the only site of metastasis [25]. Prognosis of patients with PC from GC is very poor, with median survival (MS) of 4.6 months for patients with PC only and 3.3 months for patients with PC plus other metastatic sites. Taking the hypothesis that PC is caused by serosal infiltration by the primary tumor and subsequent shedding of malignant cells into the peritoneal cavity, it is acceptable to consider advanced T and N stages as risk factors for patients with GC developing PC; risk appears to be greater if they are of younger age. Other risk factors for both synchronous and metachronous PC are signetring- cell histology of the primary tumor or linitis plastica [25].
Diagnostic laparoscopy with peritoneal cytology is the best procedure for staging advanced GC, and peritoneal cytology has been included as a staging procedure, regardless of how it is obtained (peritoneal lavage, usually during staging laparoscopy; ascitic fluid tapping if ascites is present). Positive peritoneal cytology makes the disease stage IV. Peritoneal cytology has yet to gain integration into the clinical practice of surgeons, and with missing peritoneal cytology, surgeons are accustomed to operating on GC cases without proper staging.
Cytology workup should include both Giemsa [Romanowsky, Diff Quick, May-Grunwald Giemsa (MGG), or similar methods] and Papanicolaou stains, as well as cell-block preparation (hematoxylin and eosin staining). In doubtful cases, immunohistochemical stains can be performed—usually monoclonal carcinoembryonic antigen (CEA). Real-time polymerase chain reaction (RT-PCR) to detect CK-20 and CEA messenger RNA (mRNA) expression as signs of metastasis may also help in the routine workup [26].
The pathological picture of PC from GC differs in the setting of primary advanced gastric lesion from that of PC following previous gastric resection. In the first case, the main area of neoplastic deposits is the omentum, with possible direct infiltration of the spleen or pancreas and lesser omentum, depending on location of the primary tumor. Parietal, visceral, and pelvic implants are part of the picture. Not infrequently, we have observed metastatic seeding at the ovaries (Krukenberg tumor). We also observed two cases of PC from diffuse-type GC involving the residual gastrocolic ligament and the transverse portion of the large bowel, with the dominant pathological feature represented by diffuse perineural neoplastic infiltration of the mesocolon and bowel wall (transparietal).
4.5 Peritoneal Carcinomatosis from Endometrial Cancer
PC from endometrial cancer (EC) has been rarely reported. A MEDLINE search retrieved only three articles: one concerned a wound recurrence of endometrial cancer, and two consisted of a small series of patients with PC. All patients were treated with complete CRS plus HIPEC [27–29]. The most recent series [27] involved 13 patients, of whom seven were alive after a follow-up ranging from 1.56 to 124.83 months: three had recurrence and four did not. The other published series was a small group of five patients, two of whom survived without recurrence after 2 and 3 years, and two of whom were living with recurrence after 1 and 3 years [28]. No reference was made in these articles to the overall incidence of advanced EC. However, treatment resulted in an apparent beneficial effect on survival of these patients with advanced disease. Eight cases of PC from EC have been treated in our institution with peritonectomy (PRT) plus HIPEC, with survival ranging from 12 to 95 months (see Chap. 22, Table 22.2).
Peritoneal involvement from endometrial neoplasms has a spectrum ranging from limited peritoneal areas with tiny nodules to multiple sites of neoplastic growth with nodular or plaque-like shape. Nodules can be of small or medium size and often are observed in the mesocolon, mesosigmoid, adipose tissue of splenic hilus and round ligament, and liver capsule. Plaque-like thickening is frequently seen in the subdiaphragmatic peritoneum, small- and large-bowel serosa, and subserosal adipose tissue, although nodular growth can be observed, as well. These plaque-like formations induce partial stenosis of the intestinal lumen. Macroscopic evidence of metastatic diffusion to the pericolic lymph nodes is a frequent finding, and regional lymph node stations can show metastatic nodules.
Microscopically, cases with low neoplastic burden are characterized by multiple foci of a small number of neoplastic cells, accompanied by desmoplastic stroma, and sparse in the subserosal tissue of the parietal or visceral peritoneum. Nodular and plaque-like formations show solid, papillary, cystic-papillary and, less frequently, a tubular pattern. Fibrous septa of variable thickness are present. These formations can be exophytic or invade subserosal adipose tissue. Differently from OC, involvement of muscular or more inner layers of the bowel is less frequent. Metastatic involvement of pericolic lymph nodes is usually an epiphenomenon of extensive neoplastic growth in the subserosal adipose tissue.
4.6 Peritoneal Carcinomatosis from Breast Cancer
Peritoneal involvement from previous breast cancer (BC) is a rare event. It can develop many years after the original diagnosis, with the longest reported interval being 30 years (median 18 years). Most papers in the literature are case reports. In one study [30], only 73 of 12,001 patients diagnosed with metastatic disease secondary to BC were found to have histologically confirmed metastatic disease to the GI tract and peritoneum. Twenty-three patients had GI metastasis only, with no involvement of the peritoneum. Lobular BC is prevalent in peritoneal metastasis, with rates as high as 54 % of patients compared with the 10–12 % overall percentage of lobular type in breast primaries. MS in this setting is very short, ranging from 1.5 months after treatment with chemotherapy or hormonal therapy [31] up to 26 months after surgery (mostly palliation for obstruction). A small group of five patients treated at our institution with CRS plus HIPEC achieved long-term survival, with one patient surviving up to 10 years [32].
Ductal BC is associated with a less evident macroscopic involvement. Small nodules (< 2cm in diameter) can be observed in the omentum (lesser and greater), hilus of the spleen, round ligament, parietal (parietocolic right cleft) and pelvic (pouch of Douglas) peritoneum, and subserosal adipose tissue of the left colon and mesosigmoid; single implants may be present in the cecum, mesorectum, mesentery, appendix vermiform, myometrium, paratubaric, and paraovarian tissue, with penetration of ovarian stroma.
Lobular BC shows a macroscopic picture of multiple stenosis of the large and small bowel, with tubular narrowing of the lumen due to deeper involvement of the parietal layers up to the mucosa, which appears ulcerated; round ligament, parietal peritoneum, and splenic capsule are also sites of neoplastic seeding, as are the ovaries and Fallopian tubes (ovaries show massive involvement, as in the Krukenberg type of tumor).
A panel of immunohistochemical stains is needed to confirm the diagnosis, with positive stains for CK-7 and gross cystic disease fluid protein-15 (GCDFP- 15), whereas stains for Wilms’ tumor 1 (WT1), cancer antigen 125 (CA-125), and CK-20 is negative. E-cadherin assessment is helpful in differential diagnosis between lobular and ductal BC.
4.7 Peritoneal Carcinomatosis from Pancreatic Cancer
Data regarding incidence, prognosis, and treatment opportunities of PC following pancreatic cancer are sparse in the literature. Very few studies focus on this topic. The most recent investigation is from The Netherlands [33]. The authors searched for the diagnosis of nonendocrine pancreatic cancer in the Eindhoven Cancer Registry over a period of 15 years. They found 2,924 cases, of which 265 presented with synchronous PC (mean incidence 9 %, increasing to 11 % in the last 5 years of the study). Most cases presented with metastasis in other locations. The reported MS in patients with synchronous PC was only 6 weeks. Other studies—an autopsy-based study [34] and a group of patients treated with palliative chemotherapy [35]—report incidences as high as 31 %. Another paper on disease recurrence after surgical treatment for pancreatic cancer found a PC incidence of 30 % (21/69 patients), with a grim prognosis witnessed by an actuarial 5-year survival of only 6.8 %. The main pathological characteristic independently associated with PC was invasion of the portal vein. The authors stated that PC cannot be excised surgically because cancer cells are distributed randomly across a large area of the peritoneum [36].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree