Pathology and Cytopathology
Zubair Wahid Baloch
Virginia A. Livolsi
Normal Thyroid
The normal thyroid gland is a bilobed structure, connected by an isthmus. The thyroid capsule is thin, does not strip easily, and contains sizable venous channels that become strikingly prominent when vascularity is increased. The normal gland is soft and yellowish red; the colloid gives the cut surfaces a glistening, translucent appearance. Normal thyroid weights in the United States range from 10 to 20 g. The functional unit of the thyroid is the follicle, which averages about 20 μm in diameter (1,2,3). A thyroid lobule consists of 20 to 40 follicles bound together by a thin sheath of connective tissue and supplied by a lobular artery. The thyroid follicles are formed by a single layer of low-cuboidal epithelium. The nucleus of the follicular cell is round to ovoid, sometimes irregular in shape, centrally placed, and uniform in size. The nucleolus is inconspicuous. A basal lamina envelops the entire follicle (4). Numerous capillaries and lymphatics surround the follicle. Considerable interstitial connective tissue and fat cells may be present. The follicular lumen is occupied by colloid, partly composed of thyroglobulin, which is evenly applied to the luminal cell borders. Calcium oxalate crystals are common in the colloid of adults (4,5,6).
Electron microscopy demonstrates that the normal flat- to low-cuboidal follicular cells interdigitate and overlap one another, and are intimately related to the capillaries that surround the follicle; microvilli on the apical surface are numerous near the cellular margins (7).
C-cells are intrafollicular and lie next to the follicular cells and within the basal lamina that surrounds each follicle of the normal gland. C-cells are most numerous in the central portions of the middle and upper thirds of the thyroid lobes. They are believed to originate from the last branchial pouches. C-cells are typically more numerous in infant thyroids than in adult glands (8,9).
Sizable C-cell aggregates have been observed in some adults without any known endocrinologic abnormality (10). The C-cells are polygonal to spindle-shaped, have “light” or low-density cytoplasm, and contain numerous membrane-limited cytoplasmic granules, which contain calcitonin (11). A small number of C-cells contain somatostatin (12). Guyetant et al. define C-cell hyperplasia as consisting of >40 C-cells/cm2 and the presence of at least three low-power microscopic fields containing >50 C-cells (13).
The small solid-cell nests of ovoid to spindled epidermoid cells are also considered to be of ultimobranchial origin. Typically, the nests have about the same distribution in the thyroid lobes as the C-cells (14). Tiny cysts that contain fluid, and a few mucous cells, may lie within or accompany the solid-cell nests. The so-called mixed follicles are lined by follicular cells and epidermoid cells and contain both colloid and mucoid material. The ultimobranchial structures probably also contribute a small proportion of normal thyroid follicles (14,15).
Oncocytic cells are altered follicular cells; they are enlarged, have granular eosinophilic cytoplasm, and have large, hyperchromatic, or bizarre nuclei. The cytoplasm is filled with swollen mitochondria. They are common in long-standing Graves’ disease, autoimmune thyroiditis, thyroids damaged by radiation, tumors, and some adenomatous nodules (16,17).
Small clusters of lymphoid cells are so common in the thyroid stroma that they are essentially a normal finding (18). Also present in the interstitial tissue are antigen presenting dendritic cells; these are sparse in the normal gland but increased in autoimmune thyroid disease and follicular lesions of the thyroid (19,20).
Pathologic Diagnosis of Thyroid Lesions (See Sections V and VI)
The pathologist is an integral part of the management of thyroid nodules. This includes selection of patients requiring surgery on the basis of fine-needle aspiration diagnoses and histopathologic examination of the surgically excised nodules to provide information for appropriate staging and postoperative management (21,22,23).
Fine-needle aspiration (FNA) is considered as an essential tool in providing a rational approach to the clinical management of thyroid nodules. The results of FNA can determine whether a thyroid nodule should be followed clinically or undergo surgical excision (24,25).
Every patient with a palpable thyroid nodule is a candidate for FNA and should undergo further evaluation to determine if an FNA is required. Thyroid nodules measuring at least 1.0 cm in dimension can be detected by palpation and are therefore clinically significant. However, many thyroid nodules, even though measuring >1.0 cm, may not be readily palpated due to their location in the thyroid gland (26). These and nodules measuring <1.0 cm are usually found during radiologic examination of the head and neck for non-thyroidal lesions. Thyroid nodules can be biopsied by palpation and under ultrasound; the latter is now becoming the method of choice since it provides precise information regarding the location, size, and structure of the nodule and is highly effective in getting an adequate sample for cytologic interpretation (27).
Table 16.1 the Bethesda System for Reporting Thyroid Cytopathology: Implied Risk of Malignancy and Recommended Clinical Management | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Thyroid FNA specimens can be prepared by making air-dried and alcohol fixed smears for staining with Romanowsky and Papanicolaou stains, respectively. The smears can be processed alone or with a liquid-based preparation or cellblock (28). Liquid-based preparations can be utilized either alone or as an adjunct to smears especially when on-site assessment of FNA samples is not available (24).
Thyroid FNA specimens are usually classified by employing a tiered system. Several classification schemes have been proposed by various authors based on personal/institutional experiences (24,25,29,30). In 2007, the National Cancer Institute hosted a 2-day state-of-the-art scientific meeting regarding thyroid FNA. The audiences included endocrinologists, radiologists, surgeons, and pathologists. This meeting led to various position statements on the selection of patients for thyroid biopsy, handling of thyroid FNA specimens and their cytopathologic diagnosis. At this conference many participants agreed to a six-tiered classification scheme for classifying thyroid FNA’s (Table 16.1). Each diagnostic category was assigned a risk of malignancy based on literature review along with recommendations for management (24,31,32).
The surgical pathologic assessment of thyroid specimens can be divided into intraoperative assessment/frozen section, gross, and histopathologic examination.
It has been shown that though thyroid frozen section diagnosis may be specific, it is not sensitive. In addition, deferred diagnoses at frozen section is not helpful to alter the course of surgery or alter the operative procedure (33). In lieu of frozen sections the initial approach to diagnose a thyroid nodule should be FNA (33,34,35). With an unequivocal diagnosis of malignancy the surgeon should proceed with the appropriate surgery for that malignant diagnosis. It has been shown that intraoperative assessment is most useful in cases which are diagnosed as suspicious for papillary carcinoma on FNA since the diagnosis relies on the nuclear morphology and not the finding of invasion. Thyroid nodules diagnosed as “neoplasm/suspicious for neoplasm” frozen section will not provide a definitive diagnosis and therefore should not be requested. The limited sampling at frozen section may not prove to be fruitful in finding a random microscopic focus of capsular or vascular invasion required for the diagnosis of follicular carcinoma (36,37,38).
Gross pathologic examination of a thyroid should be performed on the fresh specimen received and tumor size and appearance be documented before sections are taken for frozen section or other studies. The specimen should be oriented spatially by the surgeon. A detailed gross examination of the specimen should include: Weight and measurement, description of the external surface and the cut surface, and location, size, and physical characteristics of the nodule. The surgical margins should be highlighted with ink and the presence of gross extra-thyroidal extension should be noted. If the specimen contains regional lymph nodes, description of levels and characteristics of any grossly involved nodes should be given. The presence of parathyroid glands should be documented. The gross examination determines the number of sections to be taken for histopathologic evaluation. In diffuse lesions of the thyroid such as thyroiditis or Graves’ disease without any obvious nodules, up to three sections should be submitted from each lobe and one from isthmus. In the case of a solitary or dominant encapsulated nodule it is recommended that the entire circumference of the nodule be sectioned, whereas for a non-encapsulated nodule, one section per 0.5 cm should be submitted.
The final histopathologic report should be comprehensive and include all of the known prognostic parameters. The tumor description should include: Histologic type, number/multicentricity, size, encapsulation, presence of tumor capsule and vascular invasion, perineural invasion, and extra-thyroidal invasion. If lymph node sampling or dissection was performed, the presence of lymph node metastases, by number and size should be recorded. The identification of extranodal extension into the soft tissues should be mentioned. The number of parathyroid glands removed during surgery, if any, should be documented and their location given if possible. Additional pathologic findings in the thyroid such as nodular goiter, thyroiditis, and benign tumors should be described. Finally, based on the pathologic assessment and provided clinical information the tumor stage should be documented (39,40).
Developmental Variations
The thyroglossal tract extends in the midline from the foramen cecum at the base of the tongue to the isthmus of the normal
gland. The tract consists of connective tissue, the thyroglossal duct, lymphoid tissue, and thyroid follicles; it is attached to and may extend through the center of the hyoid bone and is intimately related to the surrounding skeletal muscle (41). Thyroid tissue may persist at the base of the tongue, and in some patients may represent the only thyroid present. The thyroglossal duct typically is a tube lined by ciliated pseudostratified epithelium. If the duct is traumatized or infected, the epithelium may be transitional or squamous, or it may be partially or completely lost and replaced by fibrous tissue. Foreign-body reaction and chronic inflammation may be conspicuous. If fluid accumulates in part of the thyroglossal duct, a thyroglossal cyst may develop (41,42). Any type of diffuse thyroid disease can involve lingual thyroid and the thyroid tissue along the tract (41).
gland. The tract consists of connective tissue, the thyroglossal duct, lymphoid tissue, and thyroid follicles; it is attached to and may extend through the center of the hyoid bone and is intimately related to the surrounding skeletal muscle (41). Thyroid tissue may persist at the base of the tongue, and in some patients may represent the only thyroid present. The thyroglossal duct typically is a tube lined by ciliated pseudostratified epithelium. If the duct is traumatized or infected, the epithelium may be transitional or squamous, or it may be partially or completely lost and replaced by fibrous tissue. Foreign-body reaction and chronic inflammation may be conspicuous. If fluid accumulates in part of the thyroglossal duct, a thyroglossal cyst may develop (41,42). Any type of diffuse thyroid disease can involve lingual thyroid and the thyroid tissue along the tract (41).
Occasionally, segments of thyroglossal duct are included within the thyroid gland proper and, rarely, may serve as the origin of an intrathyroidal cyst (41,43). Parathyroid glands, thymic tissue, tiny masses of cartilage, and tiny glands lined by ciliated cells may be seen in normal thyroid glands, presumably related to anomalies of the development of the branchial pouches (44,45,46,47).
Because of the intimate relationship that exists in the embryo between the immature thyroid tissue and the adjacent developing skeletal muscle, strips of striated muscle are occasionally included within the thyroid. Conversely, thyroid tissue may be found in perithyroidal skeletal muscle. Such nodules of thyroid tissue are particularly prominent when the gland is hyperplastic, and they should not be confused with carcinoma (48).
Groups of thyroid follicles in lymph nodes nearly always represent metastatic carcinoma. A few experienced pathologists state that normal thyroid follicles rarely occur in cervical lymph nodes. Hence, normal thyroid tissue lying only within the capsule of a node, especially if the node is located in the midline, may represent an embryologic remnant and not metastatic cancer (49,50,51).
Goiter
Goiter is a diffuse or nodular enlargement of the thyroid gland, usually resulting from a benign process or a process of unknown origin (52).
When there is a deficiency of circulating thyroid hormone because of inborn errors of metabolism (53), iodine deficiency (54), or goitrogenic agents (55), and if the hypothalamic–pituitary axis is intact, production of thyroid-stimulating hormone is increased; consequently, cellular activity and increased glandular activity and glandular mass result in an attempt to restore the euthyroid state.
Worldwide, the most common cause of thyroid hormone deficiency is an inadequate amount of iodine in the diet, leading to iodine-deficiency goiter. Other causes include inborn errors of thyroidal metabolism, dietary goitrogens, and goitrogenic drugs and chemicals.
The pathologic changes of simple nontoxic goiter include one or more of the following: Hyperplasia, colloid accumulation, or nodularity (56).
Hyperplasia represents the response of the thyroid to TSH, other growth factors, or circulating stimulatory antibodies. The hyperplasia may compensate for thyroid hormone deficiency, but in some patients, even severe hyperplasia does not produce sufficient hormonal output to avoid hypothyroidism (57).
If the deficiency of thyroid hormone occurs at birth or early in life, cretinism or juvenile myxedema may result, even though the gland is enlarged and hyperplastic; this is especially likely when an inborn error of thyroid hormone synthesis is present. A hyperplastic gland is hyperemic, diffusely enlarged, and not nodular (58,59).
The epithelium is tall and columnar; the follicles are collapsed and contain only scanty colloid. When the hyperplastic stage is extreme and prolonged, there may be confusion with carcinoma because of the degree of cellularity and the presence of enlarged cells. The nuclei are enlarged, hyperchromatic, and even bizarre. Because of follicular collapse and epithelial hyperplasia and hypertrophy, papillary changes can be seen (56,60). This pattern occurs most often in untreated dyshormonogenetic goiter (61). Recognition of the benign nature of the process is possible because all the glandular tissue is abnormal, unlike carcinoma, in which the neoplastic masses constitute one or more localized groups of abnormal cells with a background of nonneoplastic parenchyma.
Thyroid follicles may not remain in a state of continuous hyperplasia, but instead undergo involution, with the hyperplastic follicles re-accumulating colloid. The epithelium becomes low cuboidal or flattened and resembles that of the normal gland. Some follicles become much larger than normal, contain excessive colloid, and are lined with flat epithelium. The gland is diffusely enlarged, soft, and has a glistening cut surface because of the excess of stored colloid. In addition to large follicles filled with colloid, there are foci in the gland where hyperplasia is still evident. This phase of nontoxic goiter is often termed colloid goiter (62).
Patients with long-standing thyroid deficiency typically develop nodular goiters that result from overdistention of some involuted follicles, and persistence of regions of epithelial hyperplasia. The new follicles form nodules and may be heterogeneous in their appearance, in their capacity for growth and function, and in their responsiveness to TSH. The vascular network is altered through the elongation and distortion of vessels, leading to hemorrhage, necrosis, inflammation, and fibrosis. These localized degenerative and reparative changes produce some nodules that are poorly circumscribed, and others that are well demarcated and resemble true adenomas (54,63). Because the nodules distort the vascular supply to some areas of the gland, some zones will contain larger-than-normal amounts of colloid and/or iodide, and others will have relative colloid and/or iodide deficiency. Growth of goiters therefore may be related to focally excessive stimulation by TSH, stimulation by growth factors, focally abnormal iodide concentration, growth-promoting thyroid antibodies, and poorly understood intrathyroidal factors (56,60).
Nodular goiter is essentially a process involving the entire gland, but the nodularity may be asymmetric, and individual nodules within the same gland may vary greatly in size. If one nodule is much larger or more prominent than the others, distinguishing it from a true neoplasm may not be possible. Several studies have shown that about 70% of dominant nodules in nodular goiter are indeed clonal proliferations (64). The formation of cysts, hemorrhage, fibrosis, and calcification further complicates the assessment of the gland (65).
The heterogeneity of the generations of replicating follicular cells in responsiveness to outside stimuli, functional capacity,
and rate of growth, results in the formation of groups of cells that are hyperfunctional or autonomous, or both. These form “hot” nodules that may cause thyrotoxicosis (60,66).
and rate of growth, results in the formation of groups of cells that are hyperfunctional or autonomous, or both. These form “hot” nodules that may cause thyrotoxicosis (60,66).
Studies with radioactive iodine administered before operation have not always demonstrated correlations between the morphology of a nodule and its iodine metabolism (67).
Graves’ Disease
In this disorder, also termed diffuse toxic goiter, the thyroid is diffusely enlarged up to several times normal size (68).
The capsule is smooth, and the gland is hyperemic. The cut surfaces are fleshy and lack normal translucence because of the loss of colloid. If the patient is untreated, treated briefly, or receives only beta blockers, the microscopic appearance shows cellular hypertrophy and hyperplasia. The cells are tall columnar, and are thrown into papillary folds that extend into the lumina of the follicles. Blood vessels are congested (69,70). At the ultrastructural level, microvilli are increased in number and elongated, the Golgi apparatus and endoplasmic reticulum are large, and mitochondria are numerous. Infiltrates of lymphocytes lie between the follicles, ranging from minimal to extensive. T-cells predominate among the epithelial cells and in the interstitial tissue, where there are no lymphoid follicles. B-cells are numerous in the lymphoid follicles. Class II major histocompatibility complex antigens are expressed on the epithelial cells, and these epithelial cells induce the proliferation of T-cells, helping to perpetuate the process (71,72). Lymphoid hyperplasia may occur elsewhere in the body: Thymus, lymph nodes, and spleen (73).
Because nearly all patients now receive antithyroid medication and then iodide before surgery, the glands have undergone varying degrees of involution. Some appear almost normal except for numerous large follicles filled with colloid. A few papillae may remain (Fig. 16.1). The hyperemia is notably decreased, especially if there has been preoperative administration of iodide (74).
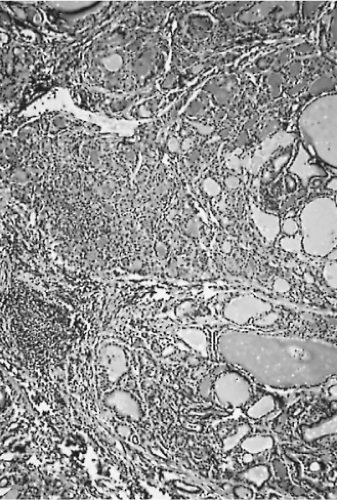 Figure 16.1 Diffuse hyperplasia of Graves’ disease. Some colloid has accumulated because of preoperative antithyroid drug therapy. |
If hyperplasia continues for many years, oxyphilic metaplasia of the cells begins to occur, the amount of stroma increases in an irregular fashion, and nodularity develops, just as in diffuse euthyroid goiter. If the process subsides spontaneously or because of maintenance on antithyroid medication, the involution may be remarkably complete, it may be irregular, or the gland may be altered by chronic lymphocytic thyroiditis (74).
Dyshormonogenetic Goiter
When an inborn error of thyroid metabolism exists, and a sufficient amount of circulating thyroid hormone is not available, the normal physiologic response of the pituitary to increase TSH causes a larger, more active thyroid, which may or may not be able to produce enough hormone to reach a normal equilibrium. If TSH stimulation is marked and prolonged, the thyroid becomes large and nodular; microscopically enlargement of follicular cells, virtual absence of colloid, and increased stroma are seen (61,75).
Large follicular cells with bizarre, hyperchromatic nuclei may be numerous. The enlarged gland, the bizarre cells, and the cellular nodules have at times been mistaken for carcinoma. Cancer can occur in a dyshormonogenetic goiter, but it is very rare (61).
Iatrogenic and Related Hyperplasias
Chronic ingestion of excess iodide, for whatever reason, occasionally leads to diffuse hyperplasia. Papillary formations and small nodules may be numerous. Infiltration of lymphocytes may occur (76).
About 3% of patients given lithium salts for a prolonged period develop goiter or hypothyroidism, or both. Patients so treated have been reported to have diffuse hyperplasia with considerable cellular and nuclear pleomorphism (77).
Bromide ingestion may lead to hypothyroidism because of loss of iodide from the gland. There are hyperplastic cells, foci of papillary proliferation, and loss of colloid (78).
Autoimmune Thyroiditis
Common synonyms for autoimmune thyroiditis include Hashimoto’s thyroiditis, lymphocytic thyroiditis, and struma lymphomatosa (79,80).
The disorder, most common in women, encompasses a spectrum of clinical and pathologic changes, ranging from an absence of symptoms to hypothyroidism and rarely, hyperthyroidism, from a large goiter to an atrophic gland, and from scattered clusters of infiltrating lymphocytes to extensive chronic inflammation and scarring with almost complete loss of follicular epithelium (79).
Various antithyroid antibodies and other immune phenomena occur, including in situ immune complex deposition and basement membrane changes in the gland, and expression of
major histocompatibility complex antigens on the thyroid cells (81). Thyroiditis may be found in the same families in which idiopathic hypothyroidism and Graves’ disease are common. It may follow typical Graves’ disease (82).
major histocompatibility complex antigens on the thyroid cells (81). Thyroiditis may be found in the same families in which idiopathic hypothyroidism and Graves’ disease are common. It may follow typical Graves’ disease (82).
The hyperthyroid variant of autoimmune thyroiditis is closely related to Graves’ disease and may be almost identical in its gross and microscopic appearance to the latter condition, suggesting that this variant may indeed be Graves’ disease. The presence of TSH-receptor antibodies in such patients would confirm the diagnosis of Graves’ disease (83,84,85,86).
If the thyroiditis is slight and focal, then the thyroid is normal in size and contains scattered infiltrates of lymphocytes, predominantly T-cells. Some of the infiltrates contain lymphoid follicular centers, mostly B-cells. The thyroid follicles involved by the infiltrates appear atrophic; they have lost part or all of their colloid (87,88,89).
Glands involved by focal thyroiditis typically are asymptomatic; therefore, the thyroiditis is discovered when thyroid tissue is surgically removed for other reasons, or the process is found at autopsy. Focal lymphocytic thyroiditis probably represents the mild or early form of autoimmune thyroiditis (79,87). When focal lymphocytic thyroiditis is more than minimal and the foci of involvement are larger and more numerous, occasional follicular cells undergo metaplasia toward oxyphilic cells. Part of a lobe sometimes may be extensively involved by lymphocytic thyroiditis, with minor changes occurring elsewhere in the gland; hence nodularity may result (79). In more advanced cases of autoimmune thyroiditis, little or no normal parenchyma is visible. The gland on gross examination is enlarged, and its cut surfaces are fleshy and pale (79).
Microscopic examination shows that many follicles are small, the amount of colloid is decreased, and infiltrates of lymphocytes, plasma cells, and macrophages are extensive. Lymphoid follicular centers/germinal centers are numerous, and their antibody-producing B-cells are polyclonal; those containing IgG are the most numerous. T-cells are most frequent among the epithelial cells and in the interstitial tissue away from lymphoid follicles (90,91,92,93). Inflammatory giant cells may be scattered through the damaged follicles; their presence should not lead the pathologist to mistake autoimmune thyroiditis for de Quervain’s thyroiditis (79,94). The amount of connective tissue in the gland often increases. Some follicular cells appear atrophic or damaged; many are hyperplastic or metaplastic (79) (Fig. 16.2). The solid-cell nests have been suggested as the origin of the latter (95,96). Possibly related rare cystic lesions have also been noted (79).
Most cases of adult hypothyroidism not related to pituitary failure, radiation, or surgical removal, probably represent an atrophic form of autoimmune thyroiditis. These glands are fibrotic and usually small, with a few nests of abnormal epithelial cells; scattered small groups of lymphocytes and plasma cells are present (97,98).
Silent Thyroiditis
Some patients with autoimmune thyroiditis have one or more episodes of painless enlargement of the gland accompanied by transient thyrotoxicosis and reduced radioiodine uptake, followed by transient and less commonly permanent hypothyroidism. The episodes often occur postpartum. Biopsies have demonstrated that the thyroid may have diffuse or focal lymphocytic thyroiditis. The entities of “silent thyroiditis” or postpartum thyroiditis have been shown to fall into the spectrum of autoimmune thyroid disease (79,99,100).
Specific Infectious Thyroiditis
Acute suppurative thyroiditis results from infection by pyogenic organisms. Tuberculosis, syphilis, parasitic infestations, and fungal infections may occur. In children, suppurative thyroiditis may occur by direct extension of infection from the pyriform sinus (101). Pneumocystis carinii thyroiditis and cytomegalovirus infection have been identified in patients with acquired immunodeficiency syndrome (102). Thyroidal infections are usually associated with the presence of the organism elsewhere in the body and occur in patients who lack a normal immune system or who are debilitated by a chronic disease (103,104,105).
Granulomatous Thyroiditis
Synonyms for granulomatous thyroiditis are subacute thyroiditis and de Quervain’s thyroiditis. This disorder, probably of viral origin, is characteristically self-limiting, lasting 1 to 3 months. Grossly, the gland is slightly enlarged and painful. The changes are usually bilateral but may be asymmetric or focal. The involved regions are firm, poorly defined, and resemble carcinoma grossly. Microscopic changes in the regions involved consist of disrupted follicles, with fragmentation of the colloid and many macrophages (106,107).
Microabscesses form, as some follicles are filled with polymorphonuclear leukocytes (Fig. 16.3). Both follicular cells and colloid are destroyed focally. Giant cells of the foreign-body type arise from the fusion of macrophages, and they lie adjacent to or surround the disrupted colloid. Fibrous tissue proliferates around the damaged follicles, and lymphocytes infiltrate the connective tissue. Because the damaged foci contain necrotic cells, macrophages, and giant cells, and because they are surrounded by proliferative connective tissue that contains lymphocytes, there is a distinct resemblance to pathologic processes characterized by the formation of granulomas (79,106).
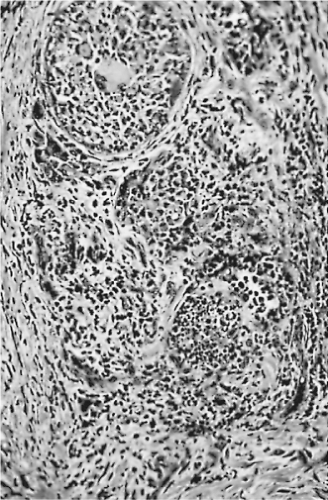 Figure 16.3 De Quervain’s thyroiditis. The five follicles shown are distended by inflammatory cells. |
The thyroid tissue between the damaged regions appears normal. Healing occurs by fibrosis and by proliferation of remaining follicular cells; new follicles appear (106).
Palpation Thyroiditis
In many thyroid specimens, particularly surgically resected ones, an occasional follicle is disorganized, with breakdown of colloid, macrophages, and foreign-body giant-cell reaction. This incidental microscopic finding is believed to be the result of palpation of the gland and hence is a posttraumatic thyroiditis (108,109).
Amiodarone Injury with Thyrotoxicosis
Administration of amiodarone may cause thyrotoxicosis. The changes in the thyroid are usually focal. Groups of follicles contain degenerated follicular cells, some follicles have lost follicular cells, and there is partial or complete loss of colloid. Zones of fibrosis are evident. The intervening thyroid tissue is normal (100,110).
Riedel’s Struma
Riedel’s struma or invasive fibrous thyroiditis is a very rare condition that represents one manifestation of a systemic collagenosis. It may include sclerosing mediastinitis, retroperitoneal fibrosis, pseudotumor of the orbit, and sclerosis of the biliary tract (111,112). The involvement of the thyroid seems to be incidental. Typically, a lobe of the thyroid and the adjacent skeletal muscle, nerves, blood vessels, and trachea are extensively replaced by dense, inflamed fibrous tissue. The mass formed is firm to hard, pale gray, and easily mistaken for cancer on clinical examination or by the surgeon at operation. Inflammatory cells, especially lymphocytes and plasma cells, are present in the dense connective tissue; angiitis usually involving veins may be conspicuous. There is no atypia of the fibroblasts or of the inflammatory cells; no oxyphilic metaplasia is found (113,114). Carcinomas with extensive fibrosis and sclerosing lymphomas should be considered in the differential diagnosis; absence of cytologic atypia is helpful in this distinction (115).
There does not appear to be any relation to other types of thyroiditis. Riedel’s struma may be unilateral, and the portion of the gland not involved by the process is normal. In some cases, remnants of a nodule or adenoma are found (114,116).
Recently, it has been suggested that Riedel’s disease belongs to the spectrum of IgG4-related systemic disease as they share the histopathologic features and both have the similar pattern of multiple organ involvement. Recognition of this link between Riedel’s thyroiditis and IgG4-related systemic disease has implication for diagnosis, treatment, and prognosis. (114,116,117).
Combined Riedel’s Struma and Fibrosing Hashimoto’s Thyroiditis
This rare condition shows simultaneous involvement of thyroid by Riedel’s struma and Hashimoto’s thyroiditis. The patients usually present with goiter and the serologic profile of Hashimoto’s thyroiditis; however, the microscopic picture is that of Riedel’s struma. Most authors believe that this simultaneous occurrence is coincidental (118,119).
Miscellaneous Disorders
Radiation Effects
Ionizing radiation delivered in small doses to the thyroid glands of infants, children, and adolescents causes a marked increase in the later incidence of benign and malignant neoplasms (120,121,122). The neoplasms begin to appear about 5 to 10 years later, but many occur decades later. Larger doses cause more numerous nodules; many of these nodules are particularly cellular, and some are atypical in their structure and cytologic features, suggesting premalignant characteristics (121,123). The cancers
that develop after small doses of radiation are mostly papillary carcinomas, are often multicentric or bilateral, and are frequently small. In addition to the nodules and neoplasms that occur, other changes are believed to be more common as well, including focal epithelial hyperplasia, chronic lymphocytic thyroiditis, oxyphilic metaplasia of follicular cells, and slight fibrosis (16).
that develop after small doses of radiation are mostly papillary carcinomas, are often multicentric or bilateral, and are frequently small. In addition to the nodules and neoplasms that occur, other changes are believed to be more common as well, including focal epithelial hyperplasia, chronic lymphocytic thyroiditis, oxyphilic metaplasia of follicular cells, and slight fibrosis (16).
Large doses of ionizing radiation initially cause injury to vessels, irregular necrosis and sloughing of the follicular epithelium, and breakdown of some follicles. Hemorrhage, edema, and small numbers of inflammatory cells appear. As the damage heals, sclerosis and dilatation of vessels occur, the fibrous stroma of the gland increases, and a mixture of atrophic, hyperplastic, and metaplastic changes take place in the follicular epithelium. In some cases, oxyphilic cells with bizarre nuclei line the follicles (98).
Amyloidosis
Black Thyroid
Prolonged therapy with tetracycline antibiotics, especially minocycline, may cause the accumulation of sufficient pigment in the follicular cells to produce a dark brown to black gland. Much of the pigment is lipofuscin, but part may be a metabolite of the drug. Rarely, there may be interference with thyroid function (126,127,128).
Neoplasms
Thyroid neoplasms demonstrate a variety of morphologic patterns that complicate their pathologic interpretation. All neoplasms that arise from thyroid epithelial cells may have some functional capacities (129). They may respond to TSH and may even produce excessive amounts of thyroid hormones (130) or, if medullary carcinoma, produce excessive amounts of calcitonin (131). Immunohistochemical evaluation is of diagnostic value in confirming the morphologic diagnosis (132,133). Localization of thyroglobulin or calcitonin aids in the classification of unusual thyroidal tumors and in providing definite identification of metastatic thyroid carcinomas (132,134).
In general, evaluation of nuclear ploidy has been of little use in assessing malignancy in thyroid tumors. Some apparently diploid tumors are malignant; some aneuploid tumors are benign (135,136,137). Measurements of steroid receptors, proliferation indices, particular antigens, and studies of the nucleolar organizing regions have provided some limited diagnostic or prognostic data (138,139,140).
Benign Neoplasms
Adenomas and Adenomatous Nodules
An adenoma or solitary adenomatous or adenomatoid nodule is defined as an encapsulated lesion having a uniform internal architecture that is substantially different from the surrounding thyroidal parenchyma, and it compresses the surrounding parenchyma (143,144). Those follicular adenomas with papillary hyperplasia should not be classified as papillary adenomas, but as papillary hyperplastic/adenomatoid nodules (145).
On gross examination, adenomas and nodules are well circumscribed and are often sharply demarcated from the adjacent tissue (Fig. 16.4). They vary in size from about 1 mm in diameter to several centimeters. The typical goitrous nodule contains so much colloid that it appears translucent, whereas the classic adenoma is cellular, fleshy, and pale. Hemorrhage, fibrosis, and cystic change may be evident in both goitrous nodules and adenomas (129).
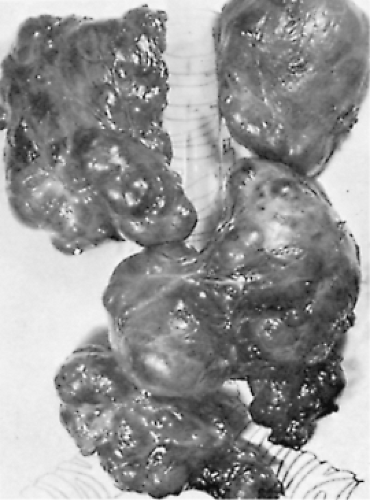 Figure 16.4 Nontoxic nodular goiter, largely mediastinal. The trachea and main-stem bronchi are represented diagrammatically. |
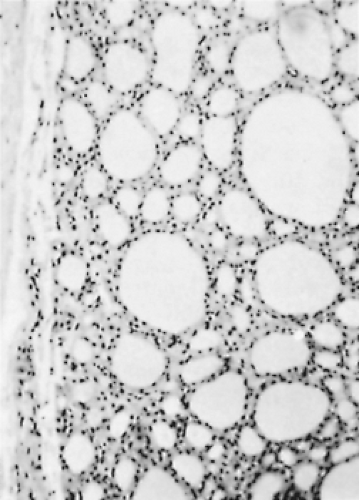
Microscopically, a typical adenomatous nodule has a varied pattern consisting of macro/large and micro/small follicles, usually with a large amount of thin/watery colloid present. Giant follicles, often irregular in shape, are common. The cells range from flat to cuboidal or columnar, and their nuclei are small, rounded, uniform, and compact. The stroma often appears loose and edematous (21,146) (Figs. 16.5 and 16.6).
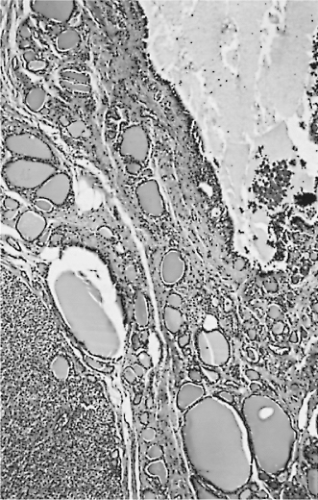 Figure 16.5 Adenomatous nodules. The lower left nodule is solid; the upper right nodule has undergone cystic degeneration. |
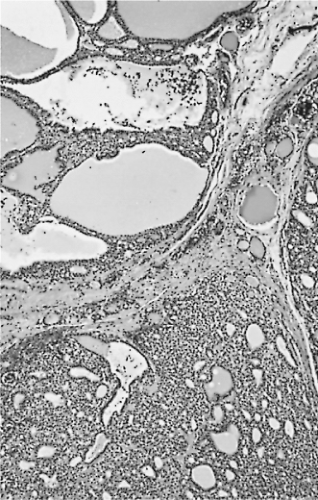 Figure 16.6 Adenomatous nodules. The nodule at the upper left contains large colloid-filled follicles. |
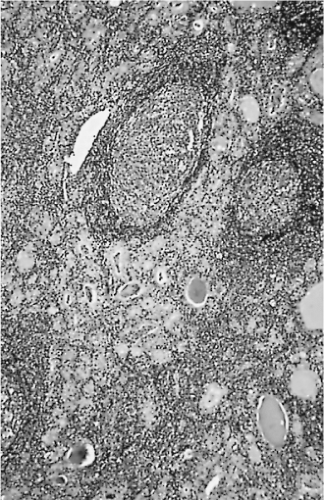
Chronic inflammation, groups of macrophages, hemo-siderin, fibrosis, and even calcification can be found. It is not unusual to see reactive/reparative changes consistent with preoperative FNA (147) (Fig. 16.7).
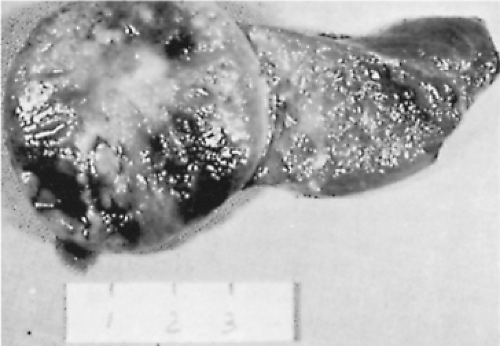 Figure 16.7 Follicular adenoma and normal thyroid tissue. A capsule is visible, and the tumor contains central fibrosis and foci of hemorrhage. |
The characteristic adenoma is encapsulated, cellular in comparison with the usual goitrous nodule, and relatively uniform in pattern. It may present as a solid mass of cells with only a hint of follicular pattern, but, more often, adenomas are composed of relatively uniform follicles (Figs. 16.8 and 16.9). Adenomas sometimes have unusual patterns and cellular features (148).
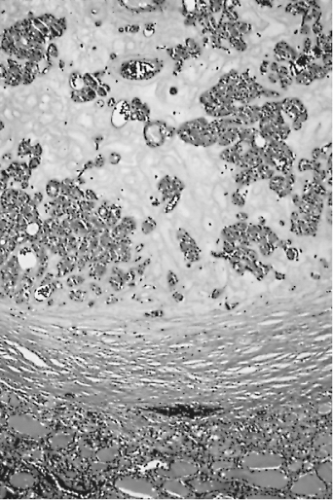 Figure 16.9 Follicular adenoma with a thick capsule. Extensive fibrous stroma is evident. Normal thyroid is at bottom. |
Some, described as atypical adenomas, are hypercellular and may contain mitotic figures, therefore resembling well-encapsulated follicular carcinoma. Such a tumor requires careful study to avoid missing a carcinoma. Other atypical adenomas are also cellular but contain spindle or polygonal cells with large and bizarre nuclei (149,150,151).
The so-called hyalinizing trabecular adenoma/neoplasm is a well-circumscribed tumor characterized by a trabecular and nesting growth pattern, usually comprised of elongated cells surrounding hyaline connective tissue. The lesional cells often contain nuclei which show intranuclear grooves and cytoplasmic inclusions (Fig. 16.10). Psammoma bodies may occur. Gradations between typical follicular adenomas, trabecular adenomas, and the hyalinizing trabecular adenomas are seen (152,153). The differential diagnosis for these neoplasms is encapsulated medullary carcinoma; the adenomas contain thyroglobulin and no calcitonin (153). A majority of authors believe that hyalinizing trabecular adenoma is benign due to its clinical behavior. However, some authors have argued against the “benign” label of this tumor and believe that it is a form
of papillary carcinoma (154,155). This is based on their similar cytologic characteristics, frequent coexistence, and similar immunoprofiles, and because some thyroid tumors with similar morphology have shown capsular and vascular invasion (155,156). By molecular analysis these tumors demonstrate RET/PTC translocations. However, to date no BRAF mutations have been reported (157,158).
of papillary carcinoma (154,155). This is based on their similar cytologic characteristics, frequent coexistence, and similar immunoprofiles, and because some thyroid tumors with similar morphology have shown capsular and vascular invasion (155,156). By molecular analysis these tumors demonstrate RET/PTC translocations. However, to date no BRAF mutations have been reported (157,158).
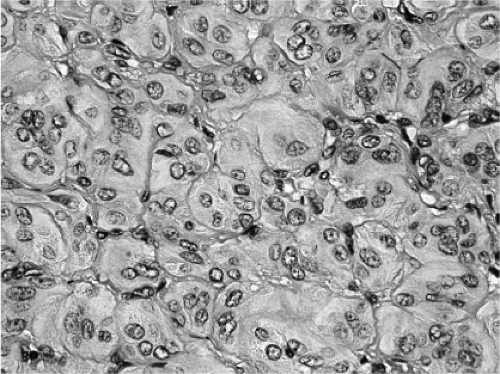 Figure 16.10 Hyalinizing trabecular adenoma/neoplasm. The tumor shows elongated cells arranged in trabecular and nesting growth pattern. |
A rare variant of follicular adenoma known as “signet-ring type” is composed of vacuolated cells in which droplets of thyroglobulin and mucin-like material are present (159). An even more uncommon adenoma is the lipid-rich cell adenomas (160,161).
Cystic change is common, especially in adenomatous nodules, and almost all the typical architecture may disappear, except for tiny remnants of the periphery. Cystic changes in nodules are frequently accompanied by the formation of papillae.
Critical examination of adenomatous nodules shows that many of these lesions are not solitary, that encapsulation and compression are inconsistent phenomena, and that their internal architecture is quite variable. Studies of clonality suggest that these are true neoplasms (64,162). Most adenomas or nodules take up little or no radioiodine and are thus “cold” on scan.
A few of these benign lesions are hyperfunctional; usually this occurs with nodules of nodular goiter rather than with a classic adenoma (67). In adolescents and young women, many of the hot, or toxic, nodules contain numerous papillae, often sufficient in number to cause a pathologist to suggest a diagnosis of papillary carcinoma (145,163,164) (Fig. 16.11).
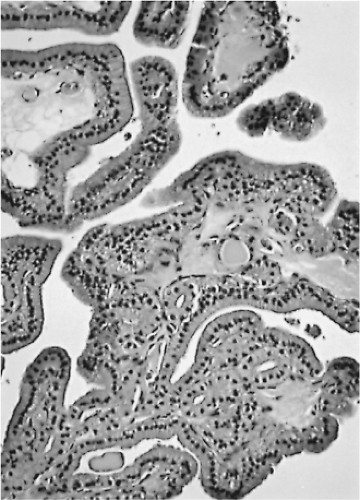 Figure 16.11 Adenoma or adenomatous nodule with papillae. The patient was 10 years old, and the nodule was hyperfunctional. The nuclei appear compact, regular, and round. |
Teratomas of the thyroid and of the tissues adjacent to the thyroid occur predominantly in infants and are usually diagnosed at the time of birth. They may become large, sufficiently so to cause dystocia by hyperextension of the neck. They are often associated with polyhydramnios. These tumors are almost always benign. Teratomas of the thyroid and the perithyroidal region are rare in adults, but can be malignant. Microscopically, the teratoma is composed of multiple elements, often with a preponderance of neural components (165,166).
Malignant Neoplasms
The most common malignant neoplasms that originate in the thyroid are the well-differentiated carcinomas of follicular epithelial origin: Most are papillary carcinomas. These constitute about 80% of thyroid carcinomas (129,167).
In predicting the prognosis in thyroid cancer, one must include the patient’s age and sex, the size of the primary tumor, the presence or absence of direct extension into the juxtathyroidal tissues, and the presence or the absence of metastatic foci (168). In some neoplasms, features such as DNA content, presence of certain cells, antigens, and molecular profile must be considered (26,169).
Most nonneoplastic diseases of the thyroid do not seem to be precursors of malignant diseases (170), with the exception that autoimmune thyroiditis may predispose to malignant lymphoma (171). An occasional adenoma or adenomatous nodule may contain a focus of papillary carcinoma when removed at operation, but this is a rare occurrence.
Anaplastic carcinomas often have arisen in goitrous thyroids, and careful examination of the resected tissues has frequently demonstrated benign tumors or well-differentiated carcinomas in close association with the anaplastic carcinoma (172). Such findings have led to suggestions that the benign tumor or low-grade carcinoma has become “transformed” into the anaplastic carcinoma (172,173).
The characteristics of well-differentiated carcinomas can be appreciated only by careful microscopic examination of multiple, well-prepared sections. Frozen sections at times may be misleading, and the surgeon must accept this limitation (33).
Papillary Carcinoma
About 80% of thyroid carcinomas are papillary carcinomas. More common in women than in men and rarely familial, papillary carcinoma occurs most frequently in those parts of the world where ample iodine is present in the diet and the environment (174,175). The association of radiation, especially low-dose external radiation in childhood, with the development of adult papillary thyroid cancer, is well documented. Studies from the areas of the former Soviet Union near the Chernobyl nuclear plant indicate an “epidemic” of thyroid carcinoma in children and teenagers following the nuclear accident and release of radioactive iodine. Virtually all of these tumors are papillary carcinomas (176).
Grossly, papillary cancers are predominantly solid, although small cystic foci may be present. A distinctly cystic character is evident in some cases, with one or more cystic spaces occupying most of the neoplasm. Bits of calcified material and crystals may be present in the cyst fluid. Papillae protruding into the cysts may be seen and at times are so numerous that portions of the cut surfaces appear granular (129,177,178,179).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree