1
Introduction
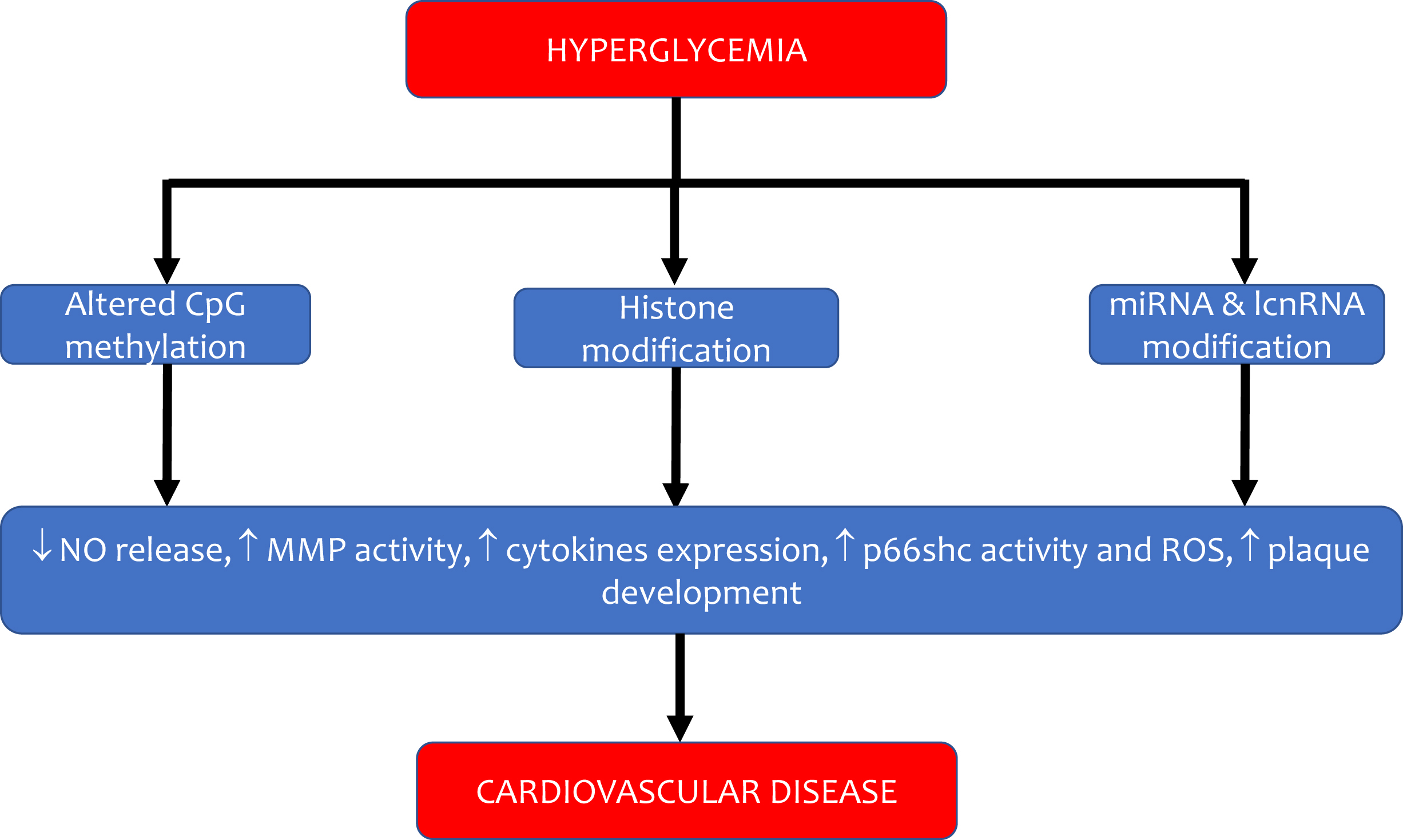
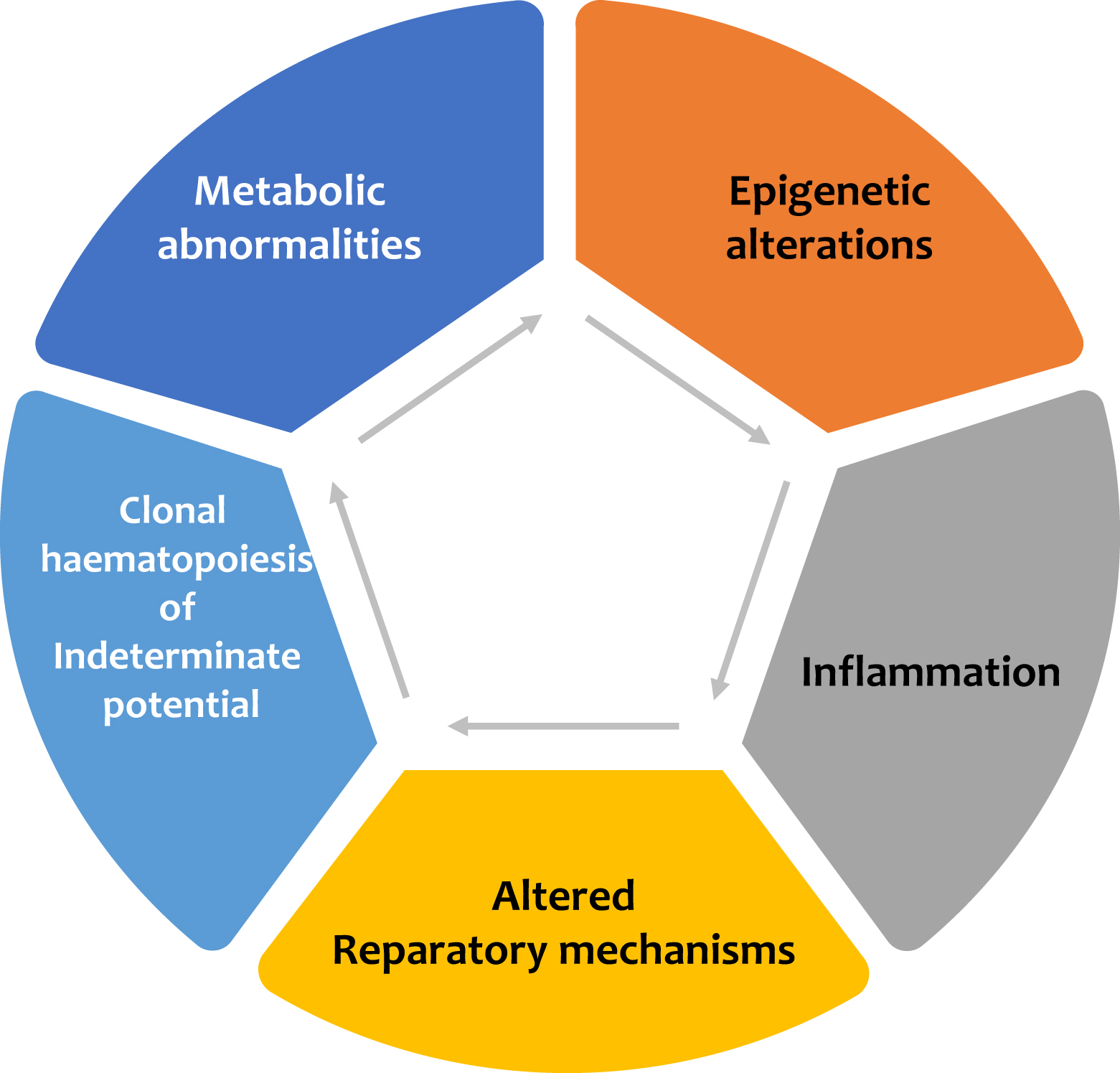
Diabetes mellitus is a significant risk factor for the development of cardiovascular disease, and it confers a two-to-fourfold increase in the risk of coronary heart disease, stroke, and peripheral artery disease. Major adverse cardiovascular events (MACEs) in patients with diabetes remain the leading cause of mortality, and the burden of cardiovascular disease attributable to diabetes has increased over the past two decades [ ]. Early studies suggested that, on average, patients with diabetes but without a previous history of myocardial infarction have a similar risk of experiencing a future cardiac event as subjects without diabetes but with a prior myocardial infarction [ ]. This high cardiovascular (CV) risk is attributed only in part to the harmful effect of hyperglycemia per se on the vascular wall: the coexistence of other traditional CV risk factors in the cluster of metabolic syndromes, such as hypertension, atherogenic dyslipidemia, central obesity, and insulin resistance, are considered crucial in the pathophysiology of vascular damage. Evidence for this comes from the data of the UK Biobank, which shows that prediabetes is independently associated with CVD [ ]. Alarming is also the increased prevalence of type 2 diabetes and associated CV risk factors among youth: among young adults with a mean age of 26 years, the event rate of heart, vascular, and cerebrovascular events was 3.73 per 1000 person-years over 13 years of follow-up since the diagnosis of the disease [ ]. These figures emphasize that the pathogenesis of CV disease in patients with diabetes starts before the clinical diagnosis of the disease. For this very reason, the risk factors leading to atherothrombosis should be corrected as early as possible. In the last decades, several important new mechanisms have been identified in the pathogenesis of diabetic atherothrombosis, which is determined not only by the negative impact of glucose on intracellular metabolism but also by its effect on other pathways related to inflammation, gene expression, and vascular regeneration ( Fig. 5.1 ).
2
Mechanisms of vascular damage in diabetes
2.1
Metabolic theory
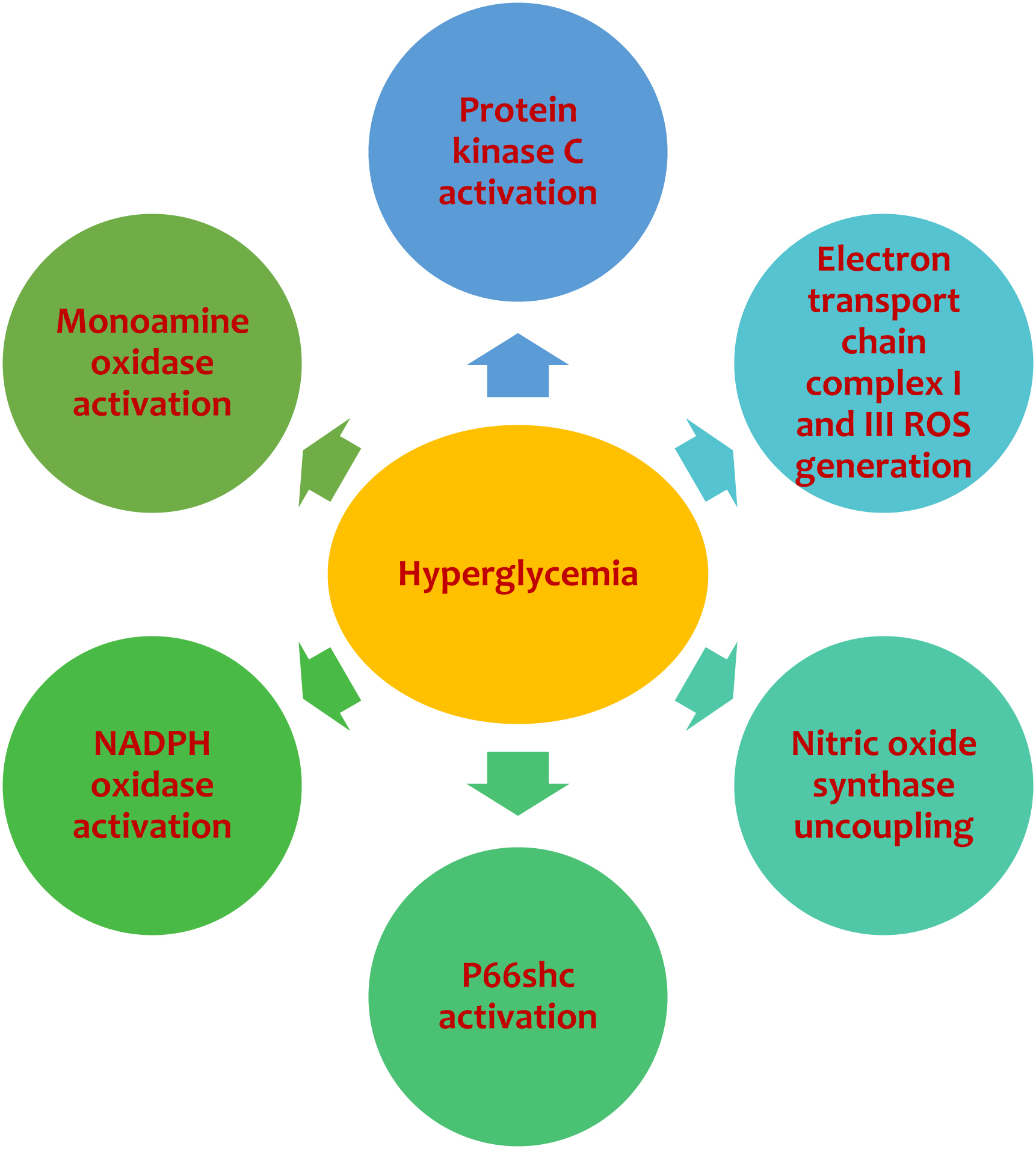
The pathophysiology of vascular damage in diabetes is complex and involves endothelial cells, vascular smooth muscle cells, and platelet function abnormalities. The endothelium is highly susceptible to glucose-induced toxicity: the exposure of these cells to hyperglycemia leads to an increased intracellular concentration of glucose and a parallel increase in reactive oxygen species (ROS) resulting from an increase in expression and activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [ ] ( Fig. 5.2 ). Elevated glucose levels result in an increased generation of diacylglycerol (DAG) via the shunting of glycolytic intermediates to dihydroxyacetone phosphate in endothelial cells. DAG activates the beta-isoform of protein kinase C (PKC) in vascular tissue and a resulting ROS production. Notably, intracellular ROS are generated in multiple compartments by several macromolecules. NADPH oxidases are located within cell membranes; however, up to 90% of generated ROS are of mitochondrial origin [ ]. In the cytosol, hyperglycemia-derived ROS can also be generated by uncoupled nitric oxide synthase and xanthine oxidases (XO). In experimental diabetes, the adaptor protein p66Shc, placed in the mitochondrial intermembrane space, and monoamine oxidase (MAO), placed in the mitochondrial outer membrane can also generate ROS [ ]. The p66Shc may generate ROS through the catalysis of electron transfer from cytochrome c to oxygen. The mitochondrial electron transport chain (ETC) is considered the most relevant source of cellular ROS. There is a close relationship between elevated glucose concentration and cellular oxidative stress due to the increased ROS generated from complexes I and III [ ]. Nitric oxide (NO), present at high concentrations in endothelial cells, stimulates mitochondrial biogenesis and inhibits complexes I and IV of the ETC. Elevated intracellular glucose concentrations are associated with changes in NO bioavailability, which subsequently leads to increased ROS production. Mitochondrial ROS induced by high glucose inhibits glyceraldehyde 3-phosphate dehydrogenase, which leads to the inhibition of glycolysis and subsequent build-up of glyceraldehyde-3-phosphate, fructose-6-phosphate, and glucose. According to the hypothesis of Brownlee and colleagues, the formation of the glycolysis products activates multiple mechanisms known to contribute to diabetic vascular complications [ ]. ROS are the inducting elements of the following pathways involved in hyperglycemia-driven vascular complications: (1) the polyol pathway; (2) the advanced glycation end-products (AGEs) formation; (3) the de novo synthesis of diacylglycerol (DAG), leading to activation of protein kinase C (PKC) isoforms; and (4) the hexosamine pathway. In the polyol pathway, the enzyme aldose reductase is activated by ROS: it catalyzes the conversion of glucose to sorbitol, leading to a shortage of the reduced isoform of glutathione, which is the primary intracellular protection against oxidative stress. AGE production of AGEs compromises extracellular and intracellular functions by forming cross-links between proteins in the basement membrane of the extracellular matrix, leading to an increase in vascular stiffness [ ]. AGE alter the biological function of intracellular proteins bind cell surface receptors, such as the endothelial receptor for AGEs (RAGE), which sparks ROS production and up-regulates the transcription of several factors such as nuclear factor-κB (NF-κB), with subsequent overexpression of many genes involved in vascular inflammation and endothelial dysfunction.
The production of ROS is also determined by the uncoupling of endothelial nitric oxide synthase [ ]. This enzyme catalyzes NO formation, which induces vasodilatation, inhibits platelet aggregation, the attachment of neutrophils to endothelial cells, the expression of adhesion molecules, and the proliferation of smooth muscle cells. In the endothelial cells exposed to elevated glucose concentrations, the expression of the eNOS is paradoxically increased rather than decreased. This condition is determined by increased endothelial levels of hydrogen peroxide, which has been shown to increase the expression of eNOS itself. eNOS is defined as “uncoupled” when, in the absence of its substrate l -arginine or the cofactor tetrahydrobiopterin (BH4), electrons normally flow from the reductase domain of one subunit to the oxygenase domain of the other subunit, are diverted to molecular oxygen rather than to l -arginine. This results in the production of superoxide rather than NO. Thus, the ability of eNOS to generate NO can be deactivated by two processes [ ]: ROS-induced oxidative changes and [ ] oxidation of BH4 to BH2. Insulin resistance and a consequent maladaptive compensatory increase in insulin concentrations is a hallmark of patients with type 2 diabetes, and it plays a relevant role in inducing vascular damage. In healthy subjects, insulin stimulates NO production by endothelial cells via the PI-3K/Akt kinase pathway; the activation of this pathway results in endothelium-dependent vasodilatation and glucose uptake in insulin-responsive tissues [ ]. Conversely, in insulin-resistant states, the PI-3K/Akt is inhibited; at the same time, the mitogen-activated protein kinase (MAPK) pathway is upregulated with a consequent triggering of proliferative and proatherogenic events in endothelial and VSMCs such as overproduction of PAI-1, endothelin, and proinflammatory cytokines.
The impact of diabetes mellitus on vascular function is not limited to the endothelium but also involves vascular muscle cells (VSMCs) and platelets. Diabetes increases the migration of VSMCs into growing atherosclerotic lesions, where they transdifferentiate into a myofibroblast-like secretory phenotype and produce an extracellular matrix [ ]. Platelet hyperreactivity is considered another pivotal factor contributing to the increased risk of coronary events in diabetic patients [ ]. Hyperglycemia alters platelet Ca2+ homeostasis, leading to cytoskeleton abnormalities and increased secretion of proaggregating cytokines. Moreover, hyperglycemia enhances the expression of both Ib and IIb/IIIa glycoproteins. Diabetes is also associated with a hypercoagulability state due to increased production of plasma factor VII, fibrinogen, PAI-1, and a reduction of endogenous anticoagulants, such as protein C and thrombomodulin. All these alterations driven by elevated glucose concentrations account for the negative impact of diabetes on vascular function and for the propensity of patients suffering from this disease to develop an early, diffuse, and severe atherothrombosis.
2.2
Epigenetic alterations
The epigenetic examines a set of reactions that regulate the activity of DNA without changes in the nucleotide sequence [ ]. The 3-dimensional chromatin conformation is regulated by DNA methylation, histone post-transcriptional modifications, and chromatin remodelers: these chromatin modifications control RNA transcription. On transcription, RNA processing and modifications represent an additional control on protein synthesis. The RNA products include long noncoding RNAs (lncRNAs), microRNAs, and circular RNAs (circRNAs), which, in turn, regulate chromatin remodeling, gene transcription, and mRNA processing and modifications. Over- or undernutrition, physical activity, and environmental factors can influence epigenetic mechanisms in adults and offspring, leading to aberrant gene expression involved in cardiovascular disorders. The heritable nature of epigenetic modifications could also predispose future generations to metabolic abnormalities, making them prone to diabetic complications later in life [ ].
The most well-known epigenetic DNA modification is methylation at 5-cytosine, essential for proper gene expression: DNA methylation with cardiovascular aging and diseases. DNA methylation at promoter regions generally leads to gene repression, whereas it might regulate transcription elongation and alternative splicing at gene bodies. Cardiovascular risk factors, such as smoking, may induce alterations in DNA methylation, which is also used as a proxy for human aging rates and is correlated with CVD incidence. The reasons for these changes in DNA methylation are unclear, but it might be correlated with the increased proinflammatory cytokines. Chromatin remodeling refers to the process by which the eukaryotic genomes wrap into nucleosome-based chromatin: one form of chromatin remodeling is the covalent modification of histones by histone acetyltransferases, deacetylases, methyltransferases, and kinases. Histone H2A, histone H2B, histone H3, and histone H4 can be modified by methylation, acetylation, ubiquitination, phosphorylation, SUMOylation, GlcNAcylation, carbonylation, and ADP-ribosylation: all these modifications can predispose to CVD [ ]. Several enzymes can modify histones by adding or removing modifications: among them, class II histone deacetylases (HDACs), which remove acetyl groups on the histones, have been linked to CVD [ ]. HDAC2 can be downregulated by oxidized low-density lipoprotein. At the same time, HDAC3 seems to have a protective role for endothelial integrity since its increased expression has been noted in areas prone to developing atherosclerosis. Its deletion of HDAC3 was linked to reduced endothelial cell survival and atherothrombosis. Increased HDAC9 was associated with enhanced expression of matrix metalloproteinases (MMP) one and two in proinflammatory macrophages. Alterations in histone modifications have been linked to cardiovascular function: the proximal promoter of the NOS3 gene, which encodes the eNOS is characterized by specific histone modifications, and changes in this profile are associated with this activation or repression of eNOS in response to environmental stimuli. The expression of proinflammatory genes, such as interleukin 6 (IL-6), and intercellular adhesion molecule 1 (ICAM-1), are regulated through DNA methylation [ ]: a significantly lower methylation level in patients with CAD has been reported compared with control subjects, suggesting an inverse association between methylation and risk for CAD, particularly AMI. In patients with diabetes, long exposure to hyperglycemia leads to long-term complications; however, meticulous glycemic control is associated with a much lower incidence or severity of complications, a phenomenon termed “metabolic memory.” In experimental conditions, metabolic memory is associated with aberrant expression of fibrotic, antioxidant, and inflammatory genes in VSMCs and endothelial cells after glucose normalization [ ]. The “metabolic memory” is strongly linked to epigenetic alterations driven by hyperglycemia. High glucose leads to alterations in DNA methylation in genes involved in the dysfunction of endothelial cells. In aortic endothelial cells, high glucose correlates with the inverse acetylation of the histone H3K9/K14 and modified DNA methylation patterns [ ]. Several histone lysine modifications have also been shown in response to transitory high glucose concentrations that may account for the transcriptional induction of the RELA gene, encoding for the p65 subunit of NF-kB. The surge in ROS can also induce the CpG hypomethylation of the p66Shc promoter and, with an increment in the H3 histone acetylation. Thus, ROS-induced epigenetic modifications are associated with higher levels of p66Shc, a mitochondrial adaptor that modulates the intracellular redox state, activates PKC, thus leading to endothelial dysfunction. A relationship between epigenetic modifications and cardio-metabolic phenotypes has been found in subclinical CVD: peripheral blood mononuclear cells were used to measure histone deacetylases (HDACs) activity and expression [ ]. Hyperglycemia increases trimethyl-histone H4 lysine 20 (H4K20me3) at retinal superoxide dismutase (sod)2, at the same time reversal of hyperglycemia failed to prevent these alterations, suggesting that epigenetic changes may be the underlying biological process of metabolic memory. Paneni and Cosentino showed that hyperglycemia, through the activation of p66shc, epigenetically regulates the promoter CpG hypomethylation. This effect leads to the maintenance of protein kinase (PKC) βII upregulation with a consequent glucose-induced ROS production and detrimental “metabolic memory” [ ]. High glucose enhances methylation of histone H3K4 and reduces methylation of H3K9 in cultured bovine aortic endothelial cells. This effect leads to chronic NF-kB activation and subsequent chronic endothelial inflammation with reduced NO bioavailability.
Noncoding RNA (ncRNA) plays an essential role in the epigenetic regulation of vascular disease and long-term diabetic complications. ncRNAs include microRNA (miRNAs), about 22 nucleotides long, but also long noncoding (lnc) RNAs, which are >200 nucleotides [ ]. miRNAs play a vital role in the epigenetic regulation of endothelial cells by downregulating the translation of target mRNAs via inhibition of posttranscriptional events, transcript degradation, or direct translational suppression. More than 1000 miRNAs have been described in mammals, some important in the CVD pathogenesis ( Fig. 5.3 ). miR-92a is a proatherogenic miRNA preferentially expressed in endothelial cells, and is highly stimulated by oxLDL: this miRNA induces chemokine and cytokine production and promotes monocyte adhesion. Abnormal expression of various miRNAs is involved in diabetic complications present in the retina, kidney, and vessel wall. miRNAs are also released within microparticles and participate in intercellular communication. We have shown that reducing circulating miR-30c-5p is associated with intima-media thickening, early atherosclerosis, and precedes plaque development by up to 11 years [ ]. We found that downregulation of miR-30c-5p occurred after treating macrophages with oxLDL via a CD36-dependent pathway involving suppressing cellular Dicer levels. In turn, miR-30c-5p reduction mediated the detrimental effects of oxLDL on IL-1β release and macrophage apoptosis, both of which were significantly prevented by a miR-30c-5p mimic. Due to oxLDL uptake via CD36 and miR-30c-5p suppression, Caspase-3, one of the predicted targets of miR-30c-5p, was induced at a transcriptional and protein level, which mediated macrophage apoptosis. Most extracellular miR-30c-5p is loaded into microparticles, which consistently transfer miR-30c-5p levels and caspase-3 to the neighboring macrophages. We found that healing of the endothelial damage was significantly impaired by miR-30c-5p reduction, thus lending support to the causal pathogenic role of the miR-30c-5p downregulation associated with plaque development in humans. Preserving miR-30c-5p levels may result in vascular protection against the early stages of atherosclerosis. Elevated glucose concentrations in human ECs provoke a surge in several miRNA, which may play an essential role in determining endothelial dysfunction through ROS formation [ ]. ROS themselves may affect miRNA levels in endothelial cells such as miR-200c, which have a critical regulatory function in proliferation, cell cycle control, and apoptosis [ ]. The overexpression of miR-200c alters the bonds between Sirtuin 1 (SIRT1), an NAD + -dependent class III histone deacetylase, with antioxidant and anti-inflammatory effects and eNOS. Therefore, miR-200c leads to an impairment of the physiological endothelial synthesis of NO and a parallel increase in ROS production.