PATHOGENESIS
Part of “CHAPTER 61 – RENAL OSTEODYSTROPHY“
OSTEITIS FIBROSA
Osteitis fibrosa results from high circulating levels of biologically active parathyroid hormone (PTH). Elevated levels of immunoreactive PTH (iPTH) and hyperplasia of the parathyroid glands are among the most consistent abnormalities of mineral metabolism in patients with chronic renal disease.1,2 The elevation of serum PTH occurs in patients with only mild (˜20%) reductions in renal function.
The principal factors involved in the pathogenesis of the secondary hyperparathyroidism of chronic renal disease include (a) retention of serum phosphorus because of a reduced glomerular filtration rate (GFR), (b) altered production of [1,25(OH)2D], (c) abnormal parathyroid growth and function, and (d) resistance to the calcemic effect of PTH.
RETENTION OF PHOSPHORUS
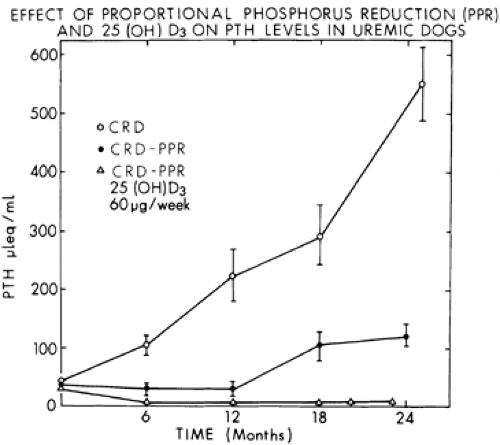
Considerable evidence supports the importance of phosphorus retention in the genesis of secondary hyperparathyroidism.3,4 and 5 In normal subjects, the oral administration of elemental phosphorus (1 g) causes an increase in serum phosphorus, a fall in serum ionized calcium levels, and an increase in serum iPTH. Moreover, the long-term administration of a high-phosphorus diet can result in parathyroid hyperplasia and increased levels of iPTH. In experimentally induced renal disease, if dietary phosphorus is restricted in proportion to the decrease in renal function, secondary hyperparathyroidism can be prevented or markedly attenuated for at least 2 years6 (Fig. 61-2). These findings have been confirmed in patients with mild to moderate renal insufficiency. Even in more advanced renal insufficiency, phosphorus restriction has resulted in substantial reductions in serum iPTH, although the levels remain above normal.
Studies have begun to refine our understanding of the mechanisms by which phosphorus retention alters parathyroid activity, as illustrated in Figure 61-3. Phosphorus directly increases the secretion of PTH7,8 and also appears to influence the growth of the parathyroid cells9 as well as to indirectly affect parathyroid
function. Furthermore, the effects of phosphorus on parathyroid cell growth have been shown to occur early in the course of experimentally induced renal insufficiency.10
function. Furthermore, the effects of phosphorus on parathyroid cell growth have been shown to occur early in the course of experimentally induced renal insufficiency.10
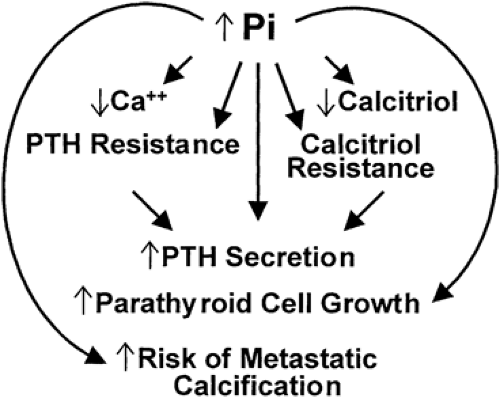 FIGURE 61-3. The consequences of hyperphosphatemia (for full description see text). (Pi, inorganic phosphate; PTH, parathyroid hormone.) |
The phosphorus retention that decreases the levels of ionized calcium (either by causing the formation of complexes with calcium that precipitate in soft tissues or, alternatively, by decreasing the production of calcitriol) also contributes to the genesis of hyperparathyroidism.11 Thus, the decreased production of calcitriol may lead to hypocalcemia by decreasing intestinal absorption of calcium or by altering PTH secretion (see later). Hyperphosphatemia also leads to resistance to the actions of PTH, as manifested by decreased release of calcium from bone, as well as to resistance to the actions of calcitriol. However, neither hypocalcemia nor hyperphosphatemia is a universal finding in patients with early renal insufficiency,12 and experimental studies have shown that hyperparathyroidism may occur without any hypocalcemia.13 Support for the role of decreased levels of calcitriol is provided by the observation that hyperparathyroidism can be prevented by the administration of calcitriol immediately after the induction of renal insufficiency.14
ALTERED PRODUCTION OF CALCITRIOL
Because the kidney is the major site of production of calcitriol14 (see Chap. 54), low levels of calcitriol may accentuate the abnormalities of mineral metabolism seen in patients with chronic renal disease. Thus, the fact that intestinal absorption of calcium is decreased in patients with far-advanced renal insufficiency is explained by the concomitant low levels of serum calcitriol. Considerable evidence exists that abnormal vitamin D metabolism contributes to the altered mineral metabolism in patients with advanced renal insufficiency. Plasma levels of calcitriol in adult patients with mild to moderate decreases in GFR (e.g., 40–60 mL per minute) are relatively normal, whereas patients with more advanced renal insufficiency have low levels of this hormone.15,16 These observations agree with studies that show that intestinal absorption of calcium is not decreased in patients with renal insufficiency until the GFR is <30% of normal.17,18 Because PTH increases the production of calcitriol, however, even normal levels of calcitriol may be inappropriately low for the prevailing metabolic state. An additional important role of low levels of calcitriol in the genesis of secondary hyperparathyroidism may be its influence on the regulation of PTH secretion at the level of the parathyroid gland.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree