ASBrS [17]
ABS [18]
Age ≥45 (IDC), ≥50 (DCIS)
Age ≥50
Size ≤3 cm
≤3 cm
Histology: IDC, DCIS
All invasive subtypes and DCIS
Negative microscopic margins
Negative surgical margins
LN negative
LN negative
No LVSI
ER positive or ER negative
In 2009, the American Society for Radiation Oncology (ASTRO) issued consensus statement (CS) recommendations, which identified the amount of scientific data in the literature for groups of patients treated with APBI subdivided by various clinical and pathologic parameters [19]. Patients were grouped into three categories: suitable, cautionary, and unsuitable (Table 27.2). The word “unsuitable,” as it is used in the ASTRO guidelines, only refers to the paucity of scientific data that is presently available for patients with these pathologic features. This designation does not necessarily indicate that it is inappropriate to treat patients with these features as part of a clinical trial or even off-trial in a properly informed patient. In addition, the “cautionary” designation only refers to the fact that limited data existed at the time the guidelines were published for the use of APBI in this subgroup of patients. Since publication, additional data have emerged (see below) which would potentially alter these guidelines for selecting patients for APBI.
Factor | Suitable | Cautionary | Unsuitable |
|---|---|---|---|
Age | 60 y/o | 50–59 | <50 |
BRCA 1 or 2 mutation | Not present | – | Present |
Tumor size | ≤2 cm | >2 cm, ≤3 cm | >3 cm |
T stage | T1 | T0 or T2 | T3 or T4 |
Margin status | Negative by ≥2 mm | Close (<2 mm) | Positive |
Grade | Any | – | – |
LVSI | No | Limited/focal | Yes |
ER status | Positive | Negative | – |
Multicentricity | Unicentric only | – | Present |
Multifocality | Clinically unifocal with total size ≤2 cm | Clinically unifocal with total size 2.1–3.0 cm | Multiple foci >3 cm apart |
Histology | Invasive ductal or other favorable subtype | Invasive lobular | |
Pure DCIS | Not allowed | ≤3 cm | If >3 cm |
EIC | Not allowed | ≤3 cm | If >3 cm |
Associated LCIS | Allowed | – | – |
N stage | pN0 (i−,i+) | – | pN1, pN2, pN3 |
Nodal surgery | SLN Bx or ALND | – | None performed |
Neoadjuvant therapy | Not allowed | – | If used |
Several studies have retrospectively categorized patients treated with APBI into the ASTRO groupings and analyzed for rates of local recurrence, axillary failure, and distant metastasis. These studies have demonstrated similar clinical outcomes following partial breast irradiation regardless of category, with similar rates of local recurrence, axillary failure, and distant metastasis for patients with pure ductal carcinoma in situ, invasive lobular carcinoma, triple-negative histology, and node-positive disease [20–23]. In a review of the ASBrS MammoSite® Registry Trial, the only factor on multivariate analysis that showed an increased propensity for local recurrence was estrogen receptor (ER)-negative status (p = 0.002), which is also true of ER-negative tumors treated with WBI [24]. Although there has been recent discussion regarding revision of these groups, no definitive plans have yet been made to change the current ASTRO CS guidelines for APBI. Results from NSABP B-39/RTOG 0413 phase III trial are not expected until at least 2015, and until that time, appropriate patient selection will continue to be on an individual basis.
Techniques for Partial Breast Irradiation
The first technique of partial breast irradiation and the one with the longest follow-up is the multi-catheter interstitial technique (Fig. 27.1). Developed as a method of boosting the lumpectomy cavity, this approach was then applied to the treatment of the peri-lumpectomy tissues alone, first with low-dose rate seeds (LDR brachytherapy) and subsequently with a high-dose rate iridium source (HDR brachytherapy) [25–28]. Most interstitial implants require 15–20 catheters and are typically arranged in two to three planes. This technique is a very versatile form of APBI, with the ability to sculpt the radiation dose as needed to treat the lumpectomy cavity and minimize radiation to other tissues (skin, normal breast, chest wall/rib, heart, lung). The most common dose and fractionation scheme for interstitial HDR brachytherapy is 3.4 Gy for ten fractions delivered twice a day with 6 h between fractions.
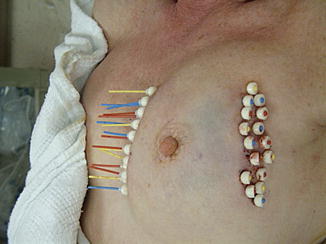

Fig. 27.1
Patient with multiple interstitial brachytherapy catheters in her breast (Image appears courtesy of Dr. L. Cuttino)
The MammoSite® Radiation Therapy System (RTS) (Hologic, Inc, Bedford, MA) was cleared by the United States FDA in 2002, dramatically changing the adoption of partial breast irradiation for breast cancer patients. Since 2002, the MammoSite® RTS (Fig. 27.2) has been used to treat over 60,000 women with early-stage breast cancer. Hattangadi has showed a dramatic 16-fold increase in the use of APBI from 2000 to the end of 2007 [29]. The main reason for this increase is the ease of insertion of the single balloon catheter and the simplicity of treatment planning.
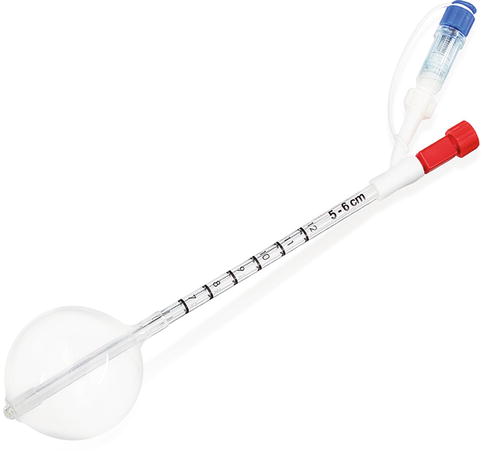

Fig. 27.2
MammoSite® single-lumen balloon brachytherapy device (Image provided courtesy of Hologic, Inc.)
However, some patients treated with the MammoSite® balloon catheter had a problem with cosmesis. Decreased cosmetic outcome after single-channel applicator-based brachytherapy was most notable in patients with decreased amount of tissue (<7 mm) from their skin to the balloon, resulting in an unwanted increase in the radiation dose to the skin. This problem was overcome by the development of second-generation multi-lumen devices that allowed for a more tailored treatment plan which could selectively adjust and minimize the radiation dose to the adjacent normal structures. The first multi-lumen device cleared by the FDA in May 2007 was the Contura® multi-lumen balloon (MLB) (Bard Biopsy Systems, Irvine, CA) (Figs 27.3a, b). The Contura® MLB has a central catheter and four offset catheters that are flexed away from the central catheter by 0.5 cm. These additional catheters allow shaping of the dose away from critical structures (skin, chest wall, lung, heart) that may be in close proximity to the lumpectomy cavity [30].
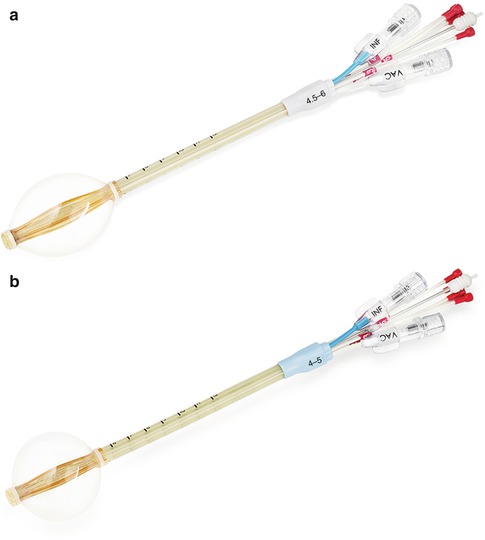

Fig. 27.3
(a, b) Contura® multi-lumen balloon brachytherapy device (Image provided courtesy of Hologic, Inc.)
Hologic developed an improved MammoSite® with multi-lumens, named the MammoSite® Multi-Lumen (ML) (Fig. 27.4) that is designed with three additional lumens offset from the central lumen by 3 mm, which also offers improved flexibility in treatment design. Prior to the introduction of multi-lumen devices, the optimal distance between the skin and balloon surface was 7 mm, which made many women ineligible for APBI. The improved dosimetric flexibility of multi-lumen balloon applicators allows the treatment of patients down to 3 mm of skin spacing (skin to balloon distance).
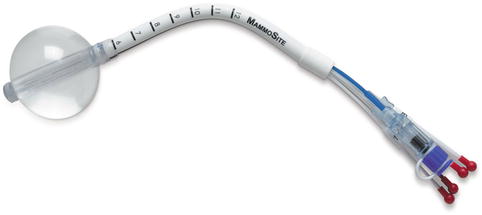

Fig. 27.4
MammoSite® ML multi-lumen balloon brachytherapy device (Image provided courtesy of Hologic, Inc.)
The first single insertion multi-lumen applicator that does not use a balloon is the Strut Adjusted Volumetric Implant® (SAVI®) (Cianna Medical, Aliso Viejo, CA) which received FDA approval in 2006 (Fig. 27.5). Unlike the balloon applicators, the SAVI® has multiple struts that project outward to create a whisk-like apparatus with each of the exterior struts in direct contact with breast tissue (similar to multiple catheter interstitial implants) [31]. This device comes in a variety of sizes with the smallest, a SAVI Mini-6, allowing brachytherapy treatment of women with small breasts or with breast augmentation (very difficult with a balloon-based device) [32].
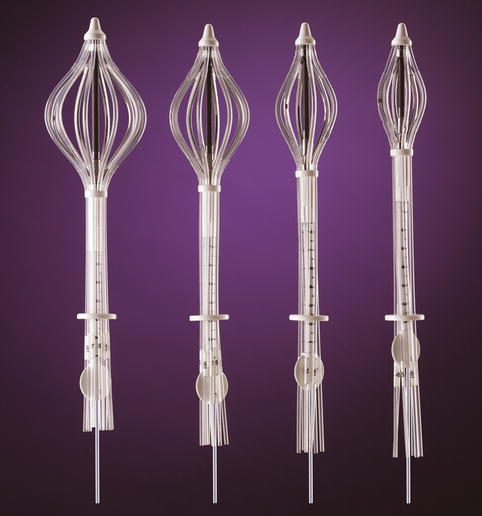

Fig. 27.5
SAVI® multi-lumen brachytherapy device (Image courtesy of Cianna medical)
Outpatient-based APBI utilizing brachytherapy is most commonly delivered via a high-dose rate (HDR) source administered using a robotic afterloader in a standard radiation vault (Fig. 27.6). An alternative to high-dose rate brachytherapy is electronic brachytherapy which is delivered with a miniaturized high-dose rate 50 kV X-ray source (Fig. 27.7) via the Axxent® brachytherapy applicator (Xoft, a subsidiary of iCAD, Inc, San Jose, CA) [33] (Fig. 27.8). An advantage of electronic brachytherapy is the reduced shielding requirements for brachytherapy treatment rooms (can be done in physician office with a lead drape over the patient). However, this system only has a single lumen and therefore minimal ability to shape the dose away from normal tissue, with further theoretical concerns about the radiobiologic effect (RBE) of low-energy X-rays. This will need to be closely monitored as radiation centers gain experience with this system. The Xoft Axxent balloon-based system is also used for another APBI technique, intraoperative radiotherapy (IORT), as discussed in the following section.
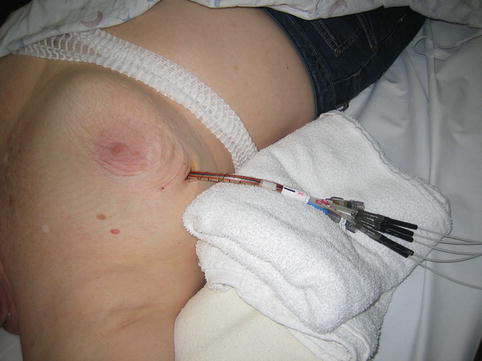
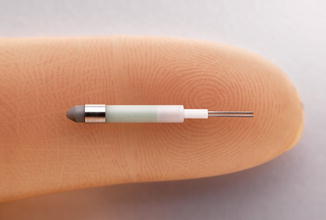
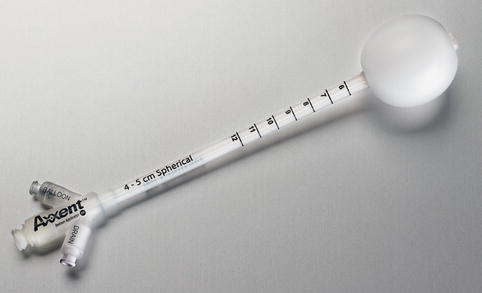

Fig. 27.6
Afterloader connected to a multi-lumen applicator for high dose rate brachytherapy (Image appears courtesy of Dr. L. Cuttino)

Fig. 27.7
X-ray source for Xoft brachytherapy system (Courtesy of Xoft, a subsidiary of iCAD, Inc.)

Fig. 27.8
Axxent single-lumen balloon catheter (Courtesy of Xoft, a subsidiary of iCAD, Inc.)
The most widely used method of accelerated partial breast irradiation is three-dimensional conformal radiotherapy or 3D-CRT. Initially introduced in 2003 [34], this method uses a standard linear accelerator with between three and five noncoplanar external radiotherapy segments to deliver the accelerated course of radiotherapy to the area surrounding the lumpectomy cavity (Fig. 27.9). There are limitations to the 3D-CRT technique including the need to treat a larger volume of breast (and normal) tissue surrounding the lumpectomy cavity due to variability with setup and respiration (neither of which are an issue with internal/HDR brachytherapy) as well as an increased dose to the contralateral breast, heart, and lungs. The treatment is delivered two times per day, similar to conventional brachytherapy, but with a slightly larger dose per fraction (3.85 Gy) to a total prescription dose of 38.5 Gy to account for the lack of heterogeneity using this technique.
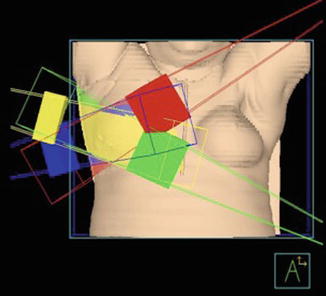

Fig. 27.9
Example of four-field three-dimensional conformal radiation therapy (3D-CRT) (Image appears courtesy of Beaumont Health System)
Intraoperative APBI
In Europe, several centers have developed intraoperative radiation therapy (IORT), which delivered a single dose of radiation immediately following lumpectomy but prior to wound closure. One technique utilizes a standard linear accelerator built into the operating room to deliver a single 21 Gy dose of electrons [35]. Intraoperative partial breast irradiation has the distinct advantage of visualizing the tumor bed at the time of surgery; however, final pathologic assessment of the margins and lymph nodes is not available at the time of treatment. Advocates for IORT promote the potential benefit of treating residual tumor cells prior to the onset of hypoxia, which can occur following breast surgery. In addition to the Xoft® Axxent® balloon-based system, another form of intraoperative RT uses a high-dose rate, low-energy X-ray source (50 kV) placed directly into the lumpectomy via a collimator with a sphere on the end (INTRABEAM® System). There is a randomized, phase III clinical trial of targeted intraoperative radiotherapy versus whole breast radiotherapy (TARGIT-A trial) that has accrued to completion and awaits long-term follow-up data on these patients. Preliminary results, published in 2010, showed non-inferiority of the IORT arm as compared with whole breast irradiation; however, an update provided at the 2012 San Antonio Breast Cancer Symposium suggested an increased rate of ipsilateral recurrence for patients treated with IORT [36]. The most recent publication of the TARGIT data suggests that excess local recurrences may primarily occur when the single-fraction technique is used at an extended time from the original surgery [37]. Xoft has also initiated a prospective, multicenter, non-inferiority study utilizing the Xoft® Axxent® System for IORT which will enroll 600 patients; the results of this study are pending at this time.
Other APBI Techniques
Novel concepts that are being studied in partial breast irradiation include LDR seed implantation into the breast [38], intraoperative pre-lumpectomy radiation therapy [39], stereotactic radiation therapy utilizing a CyberKnife® [40], and proton therapy. Proton therapy for partial breast irradiation has been investigated at several centers including Massachusetts General Hospital, M.D. Anderson Cancer Center, and Loma Linda University [41, 42]. An initial publication from Massachusetts General by Kozak et al. suggested a higher than expected acute skin toxicity with proton-based APBI; however, a separate phase II trial conducted at Loma Linda University did not report this same toxicity pattern. The unexpected toxicity produced within the protocol at Massachusetts General was likely due to the use of a single gantry angle as opposed to two to three tangents. Ongoing prospective study of particle therapy for partial breast irradiation with long-term follow-up is needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree