Parathyroid disease, syndromes and pathophysiology
- William B. Inabnet
- James A. Lee
- Barnard J.A. Palmer
- James A. Lee
Introduction
Hyperparathyroidism is a disease characterised by elevated serum calcium and inappropriately elevated parathyroid hormone (PTH) levels, which occurs with a prevalence of 3 per 1000 in the general population. The modern era of treating parathyroid disease began in 1925, when Mandl performed the first parathyroidectomy in a patient with severe bone disease. Early in the history of hyperparathyroidism, patients presented with advanced clinical disease, including fractures, skeletal deformities, kidney stones and kidney failure. The discovery of the peptide PTH in the early 1970s, coupled with the development of a chemical analyser to measure calcium, permitted the biochemical diagnosis of hyperparathyroidism much earlier in the disease course.
During this era, bilateral neck exploration was the standard approach, resulting in a cure rate ranging from 92% to 96% when performed by a skilled surgical team.
Over the last 20 years, the treatment of hyperparathyroidism has experienced a dramatic change with the development of new technology to permit accurate preoperative localisation of abnormal glands, and intraoperative confirmation of the completeness of parathyroid resection.
Embryology and anatomy
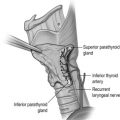
In order to successfully diagnose and treat disorders of the parathyroid glands, a keen understanding of parathyroid embryology and anatomy is essential. The parathyroid glands are small, brownish-tan glands located in the space around the thyroid gland. During the fifth week of foetal development, the inferior parathyroid glands arise from the dorsal aspect of the third pharyngeal pouch. Following development of the thymus from the ventral aspect of the third pharyngeal pouch, the inferior parathyroid glands and thymus descend in a caudal and medial direction to rest in the inferior neck and thorax respectively. The superior parathyroid glands arise from the dorsal wing of the fourth pharyngeal pouch and descend in a caudal direction with the thyroid gland.
Because of the longer pathway of descent, the inferior parathyroid glands have a higher variability of location compared with the superior parathyroid glands, an observation that is important during parathyroid surgery.
In an autopsy series of 503 human subjects, Akerstrom et al. showed that four parathyroid glands were present in 84% of cases, whereas 3% of patients had only three glands and 13% had supernumerary glands. The presence of missed hyperfunctioning supernumerary glands is an important but infrequent cause of persistent hyperparathyroidism and should be considered in all cases of persistent disease. In 80% of cases, the location of the inferior and superior glands is symmetrical when compared with the glands on the contralateral side of the neck. The superior parathyroid glands are most commonly found immediately superior to the junction of the recurrent laryngeal nerve and the inferior thyroid artery and can be located inside the thyroid gland in 0.2% of cases.
Approximately 50% of inferior parathyroid glands are located in the vicinity of the inferior pole of the thyroid gland and about 30% are found in the thyrothymic ligament.
Calcium and parathyroid hormone (PTH) regulation
The parathyroid glands play a central role in regulating serum levels of calcium through a complex feedback loop involving PTH, serum ionised calcium levels and vitamin D. The key organ systems involved in this process include the parathyroid glands, gastrointestinal tract, kidneys and skin. Although multiple factors influence parathyroid function, it is now clear that calcium is the single most potent stimulator of PTH release. Calcium-sensing receptors (CSRs), which are located on the surface of the parathyroid chief cells and are coupled with a G-protein receptor, are able to detect minuscule changes in serum levels of extracellular ionised calcium. When serum levels of calcium decrease the CSRs are activated, thereby stimulating the synthesis and release of PTH. In primary hyperparathyroidism (PHP), the set point of the CSRs is adjusted upwards, probably through a mutation of unknown aetiology, causing the parathyroid chief cell to ‘believe’ that serum calcium levels are low when in fact they are not. As a result of this alteration in the CSR set point, the parathyroid chief cell increases production of PTH, ultimately leading to hypercalcaemia. Calcium-sensing receptors are also present in other tissues such as the kidneys and gastrointestinal tract, where calcium homeostasis is influenced. In the kidney, the CSRs regulate renal calcium excretion and influence the transepithelial movement of water and other electrolytes. In the gastrointestinal tract, CSRs are present in the gastrin-secreting G cells and acid-secreting parietal cells, thereby providing a molecular link between hypercalcaemia and acid hypersecretion. These facts also underscore the complexity of calcium homeostasis in influencing cellular function throughout the body.
PTH is an intact 84-amino-acid peptide with amino and carboxy terminals. Production of PTH begins in the endoplasmic reticulum of the parathyroid chief cells as a 115-amino-acid molecule, which undergoes a series of cleavages before being released from the cytoplasm as the biologically active (1–84)PTH molecule. The circulating (1–84)PTH molecule, which has a half-life of 3–5 minutes in patients with normal renal function, is initially cleaved in the liver, yielding an inactive C-terminal fragment, which is ultimately cleared by the kidneys. The N-terminal fragment is the part of the peptide that is responsible for the biological activity of PTH in peripheral tissues.
PTH acts directly on the kidneys, bone and gastrointestinal tract to activate several intracellular second messengers, including cyclic AMP and calcium. In the kidneys, PTH increases serum calcium levels by acting on the renal tubule to increase resorption of calcium and to increase the hydroxylation of 25-hydroxyvitamin D to the biologically active 1,25-dihydroxyvitamin D. PTH also stimulates the renal tubular secretion of phosphate and bicarbonate. In the bone, PTH acts on osteoblasts and osteoclasts to increase bone turnover, thereby providing a large source of calcium for the extracellular space.
Vitamin D is a fat-soluble vitamin that is prevalent in dairy products. After being absorbed by the gastrointestinal tract, it is hydroxylated in the liver to become 25-hydroxyvitamin D, which in turn is hydroxylated in the kidneys to become 1,25-dihydroxyvitamin D. The latter plays an important role in calcium homeostasis by increasing the resorption of phosphorus in the kidneys and increasing the absorption of calcium from the gastrointestinal tract. Calcitonin, which is synthesised by the parafollicular C cells of the thyroid gland, acts as the physiological antagonist to PTH. Calcitonin decreases serum levels of calcium by decreasing bone turnover and in fact can be used to treat patients in hypercalcaemic crisis.
Primary hyperparathyroidism
Incidence
Early in the history of PHP, patients presented with manifestations of severe hypercalcaemia and advanced disease, but the true incidence of hyperparathyroidism was not known due to the inability to routinely measure serum calcium levels. The development of the automated serum chemical analyser and the practice of widespread biochemical screening permitted the detection of mild increases in serum calcium levels, thereby allowing earlier recognition of abnormalities in calcium homeostasis.
Multiple factors influence the incidence of PHP, including the region of the world under evaluation, the nutritional status of the studied population, iatrogenic factors and the availability of routine biochemical screening.
In the 1970s, there was a dramatic fivefold increase in the incidence of PHP, largely due to the ‘catch-up’ effect of identifying patients who had PHP prior to the development of the automated calcium analyser. During the 1980s, the incidence of PHP in North America actually decreased as the impact of the ‘catch-up’ effect levelled off.
The number of patients with a history of irradiation to the head and neck region for benign disorders decreased in the 1980s, which may also have contributed to the decreased incidence of PHP, as head and neck irradiation is a known risk factor for parathyroid hypersecretion.
PHP occurs more frequently in women than men, but the overall incidence increases with age in both sexes. In North America, the incidence of PHP in the general population is 4.3 per 1000, whereas in Europe the incidence is 3 per 1000. In women aged between 55 and 75 years, the incidence of PHP is 21 per 1000. Possible explanations for the increased incidence with age include the lower rate of biochemical screening in patients less than 50 years of age and the increased use of bone density measurements in postmenopausal women as a routine part of healthcare screening. The detection of osteopenia and/or osteoporosis that is out of proportion to age-matched controls often leads the clinician to measure serum calcium and PTH levels, thereby identifying hyperparathyroidism as the cause of increased bone loss. Vitamin D deficiency also influences the true detected incidence of PHP as this condition may cause serum calcium levels to be normal in patients with hyperparathyroidism. For example, the incidence of vitamin D deficiency in southern Europe is high, leading to an underestimation of the true incidence of hyperparathyroidism in this region of the world.
Clinical manifestations
The clinical presentation of patients with PHP is highly variable, ranging from none to profound symptoms of hypercalcaemia, such as excessive thirst, dehydration, kidney stones, muscle weakness and pathological fracture. Generally, the clinical manifestations of PHP can be broadly classified by organ system ( Box 1.1 ). Since many of these symptoms overlap with other clinical conditions, particularly in the elderly, the diagnosis of hyperparathyroidism is often delayed until hypercalcaemia is recognised on biochemical screening. Often the presence of a classic symptom, such as nephrolithiasis, will lead the astute clinician to assess the patient for PHP. By far, fatigue is one of the most common symptoms of hyperparathyroidism, being present in > 80% of patients. Numerous studies have shown that a high percentage of patients that are thought to be asymptomatic actually have occult symptoms attributable to PHP.
Gastrointestinal
- •
Nausea/vomiting
- •
Epigastric pain
- •
Pancreatitis
- •
Peptic ulcer disease
- •
Anorexia
- •
Weight loss
- •
Constipation
Cardiovascular
- •
Hypertension
- •
Shortened Q–T interval, wide T wave
- •
Bradycardia
- •
Heart block
- •
Lethal arrhythmias
Renal
- •
Renal colic
- •
Polyuria/oligurla/anuria
- •
Thirst/dehydration
- •
Renal failure
Neuropsychiatric
- •
Anxiety
- •
Headaches
- •
Dementia/paranoia
- •
Confusion
- •
Depression
- •
Muscle weakness
- •
Hyporeflexia
- •
Ataxia
- •
Coma
Miscellaneous
- •
Visual changes
- •
Band keratopathy
- •
Conjunctivitis
- •
Myalgia
- •
Pruritus
There are numerous medical conditions that are associated with and/or exacerbated by PHP, including hypertension, diabetes, pancreatitis, nephrolithiasis, gout and peptic ulcer disease.
Diagnosis
Prior to the 1970s and the advent of routine serum calcium measurements as part of the basic metabolic profile, the diagnosis of PHP was made primarily on clinical findings. Walter St Goar immortalised this constellation of findings in the mnemonic ‘bones, stones and groans’. However, with routine serum calcium measurements, an elevated serum calcium level has become the most common presentation. PHP is confirmed by elevated serum calcium and serum PTH levels and can be suggested by other laboratory values (see below):
- •
Elevated serum calcium. While a useful screening tool, many conditions can lead to inaccuracies in the measured total serum calcium levels. For example, hypoalbuminaemia and acidosis can create ‘normal’ serum calcium levels. Given these variables, many groups favour measuring the ionised serum calcium level instead. Monchik found in a number of series that an elevated serum ionised calcium correlated better with the presence of PHP as confirmed by surgery.
- •
Elevated serum PTH. Current antibody-driven assays for serum intact parathyroid hormone (iPTH) levels are highly accurate.
- •
Chloride:phosphate ratio. A recent retrospective study suggests that a chloride:phosphate ratio ≥ 33 is indicative of PHP in both hypercalcaemic and normocalcaemic patients.
- •
Hypercalciuria. The presence of hypercalciuria rules out benign familial hypercalcaemic hypocalciuria, which can mimic PHP.
- •
Hypophosphataemia. Due to the decreased resorption of phosphate by the renal tubule, phosphate levels decrease in approximately 50% of patients with PHP.
Normocalcaemic hyperparathyroidism
There is a small subset of patients with PHP who present with normal or only intermittently elevated calcium levels. Mather first described normocalcaemic hyperparathyroidism in 1953 in a woman who presented with osteitis fibrosa cystica. Since that time, this variation of PHP has been an infrequent but recognised entity. While still uncommon when compared with hypercalcaemic PHP, recent population studies have shown that this variant of the disease may be more prevalent than previously believed and that improved screening may help identify mildly symptomatic or asymptomatic patients.
The exact biochemical mechanisms of normocalcaemic PHP remain elusive. Some investigators postulate that the normocalcaemic variant of PHP represents an early or preclinical phase that progresses to typical hypercalcaemic PHP. Others have found distinct differences in the biological response to PTH in patients with normocalcaemic vs. hypercalcaemic hyperparathyroidism. For example, Maruani et al. found that patients with normocalcaemic hyperparathyroidism displayed a resistance to the renal and bony effects of PTH as measured by a lower fasting urine calcium excretion and renal tubular calcium resorption, as well as lower values of markers of bone turnover.
While most patients with normocalcaemic PHP present with nephrolithiasis, recent data show that other classic constitutional symptoms are just as prevalent in normocalcaemic patients as in hypercalcaemic PHP patients, suggesting that there is a larger unidentified population with PHP.
The majority of patients with normocalcaemic PHP present with renal calculi and hypercalciuria. However, the most common cause of renal calculi and hypercalciuria is idiopathic hypercalciuria (IH). To further confound the matter, some variants of IH have elevated PTH levels. It is vitally important to distinguish between these two entities since surgical parathyroidectomy effectively cures normocalcaemic PHP, whereas postsurgical IH patients continue to form stones.
Many tests are helpful in differentiating between the two diseases, but none has been shown to be conclusive enough to be used in isolation. The best diagnostic yield is to use two or more tests in combination:
- •
Thiazide administration. Administration of thiazide diuretics leads to a decrease in urinary calcium excretion. Patients with normocalcaemic PHP will have persistently elevated PTH levels, whereas those with IH will have a normalisation of PTH.
- •
Phosphate deprivation. After restricting phosphate to 350 mg/day and administering 650 mg of aluminium hydroxide four times a day (while on a normal calorie and normal calcium diet), serum calcium and phosphorus levels are checked every day for 4 days. Patients with subsequent hypercalcaemia or persistent hypercalciuria usually have normocalcaemic PHP. This test is no longer used routinely.
- •
Calcium loading test. After administration of either 350 or 1000 mg of oral calcium, serum calcium and urine calcium are measured. Patients with normocalcaemic PHP have a significant increase in serum calcium (due to increased intestinal absorption) and an increase in urine calcium excretion, whereas intestinal absorption of calcium varies widely in patients with IH. In a recent study, after administration of 1 g of oral calcium, the combined parameters of (i) circulating PTH nadir (pg/mL) × peak calcium concentration (mg/dL) and (ii) relative PTH decline/relative calcium increment diagnosed normocalcaemic PHP with 100% sensitivity and 87% specificity. Furthermore, calcium loading suppressed urinary cyclic AMP but did not suppress PTH levels below 70% of baseline.
- •
Serum ionised calcium. An elevated ionised calcium, in conjunction with an elevated PTH, is increasingly gaining acceptance as an excellent means of distinguishing normocalcaemic PHP from IH.
As mentioned previously, the mainstay of treatment for normocalcaemic PHP is operative parathyroidectomy.
Hypercalcaemic crisis
Hypercalcaemia is seen in approximately 0.5% of the general population and up to 5% of the hospital population. The majority of cases of hypercalcaemia are classified as mild to moderate (< 12 or 12–14 mg/dL respectively) and the patient is asymptomatic. This group responds to dietary measures and treatment of the underlying aetiology. However, a subset of patients will present in hypercalcaemic crisis, with serum calcium > 14 mg/dL, and are severely symptomatic. These patients require hospitalisation and aggressive reduction of serum calcium. Fortunately, except in cases of malignancy, treatment for hypercalcaemia is typically successful.
Since the calcium ion plays a crucial role in membrane potentials throughout the body, the symptoms of hypercalcaemia are varied and potentially life-threatening. The classic presentation of severe hypercalcaemia includes acute confusion, abdominal pain, vomiting, dehydration and anuria. In addition, patients may develop lethal arrhythmias due to decreased conduction velocities and shortened refractory periods, which manifest on an electrocardiogram as a prolonged P–R interval, a shortened Q–T interval, and arrhythmia. Hypercalcaemic crisis is the most extreme form of hypercalcaemia and is defined as severe hypercalcaemia in association with profound dehydration and obtundation. At serum calcium levels of 15–18 mg/dL, coma and cardiac arrest may occur.
The most common aetiology of hypercalcaemia in non-hospitalised patients is PHP, while malignancy accounts for almost two-thirds of the hypercalcaemic inpatient population. It is crucial to identify the underlying cause of hypercalcaemia in order to effectively and definitively address the acute event. Box 1.2 lists the differential diagnoses for hypercalcaemia. The treatment of severe hypercalcaemia revolves around aggressive rehydration, increasing renal excretion of calcium, blunting of calcium release from skeletal stores, and treating the underlying cause of the hypercalcaemia.
Malignancy
- •
Solid tumour (parathyroid hormone-related peptide mediated): lung, kidney, squamous cell carcinoma of head/neck/oesophagus/female genital tract
- •
Metastases (osteoclastic lesions): breast, prostate
- •
Haematological: multiple myeloma, lymphoma, leukaemia
Hyperparathyroidism
- •
Primary hyperparathyroidism
- •
Familial: multiple endocrine neoplasia (MEN) types 1 and 2, benign familial hypocalciuric hypercalcaemia, idiopathic hypercalcaemia of infancy
- •
Lithium
Increased bone turnover
- •
Vitamin A intoxication
- •
Thiazide diuretics
- •
Hyperthyroidism
- •
Immobilisation
- •
Paget’s disease
Excess vitamin D
- •
Vitamin D intoxication
- •
Increased 1,25-dihydroxylated vitamin D: granulomatous disease
Renal failure
- •
Milk alkali syndrome
- •
Secondary hyperparathyroidism
- •
Aluminium intoxication
Miscellaneous
- •
Addisonian crisis
- •
Laboratory error: haemoconcentration, hypoproteinaemia
The primary goal of treatment is to achieve adequate volume resuscitation, which in turn increases calcium excretion in the kidneys. Patients are invariably dehydrated due to poor oral intake and vomiting. The resultant decrement in glomerular filtration rate leads to a decrease in renal excretion of calcium. Typically, 200–500 mL/h of normal saline are given to maintain urine output above 100 mL/h, with the caveat that comorbidities may limit the rate of resuscitation. Using normal saline lends substrate for the resultant natriuresis. Once the intravascular volume is restored, loop diuretics such as furosemide may be given to enhance calciuresis by inhibiting calcium resorption in the thick ascending limb of the loop of Henle. During the resuscitative phase, the patient must be monitored closely for signs of fluid overload, hypokalaemia and hypomagnesaemia. Serum calcium levels can be reduced by 1.6–2.5 mg/dL within 24 hours by volume repletion and loop diuretic administration alone. However, when serum calcium exceeds 12 mg/dL or hypercalcaemia is caused by malignancy, intravenous fluids and diuretics alone are usually insufficient to normalise calcium levels.
Numerous agents are available to blunt the release of calcium from bone resorption and treat the underlying disease. Table 1.1 provides an overview of agents available to combat hypercalcaemia and their relative strengths and weaknesses.
- •
Bisphosphonates: pamidronate 60–90 mg i.v. Bisphosphonates are pyrophosphate analogues that are concentrated in areas of high bone turnover and inhibit osteoclast activity. Endogenous phosphatases cannot hydrolyse the central carbon–phosphorus–carbon bond, making this drug stable in vivo. Bisphosphonates should be given intravenously due to their poor absorption by the gastrointestinal tract. In the USA, only etidronate (first generation) and pamidronate (second generation) are approved for use in treating hypercalcaemia. Pamidronate has widely supplanted etidronate as the bisphosphonate of choice due to its faster onset, increased duration of action, increased efficacy and minimal adverse effect on mineralisation. One dose of intravenous pamidronate normalises serum calcium for 10–14 days in 80–100% of patients with hypercalcaemia of malignancy. Newer, more potent generations of bisphosphonates may replace pamidronate as the standard as more clinical data become available.
- •
Calcitonin: salmon calcitonin 4–8 U/kg s.c./i.v. Calcitonin diminishes osteoclast activity and increases calciuresis within minutes of administration. However, the duration of action is limited to only a few days. Calcitonin therapy only rarely results in normocalcaemia. Tachyphylaxis limits the long-term use of calcitonin. Currently, calcitonin is used primarily as an immediate hypocalcaemic agent that temporises until the more sustained effects of other agents begin.
- •
Gallium nitrate: 200 mg/m 2 i.v. q.d. for 5 days. Gallium nitrate inhibits bone resorption by reducing the solubility of hydroxyapatite crystals. This drug induces normocalcaemia within 2–3 days that lasts for 5–6 days in approximately 75% of patients. The use of gallium nitrate has been limited by its nephrotoxicity, the need for continuous infusion and lack of clinical data.
- •
Plicamycin: 25 μg/kg. Plicamycin is an osteoclast cytotoxin originally used in chemotherapy. Due to its serious side-effects (hepatic, renal and bone marrow toxicity), plicamycin is reserved for patients who fail bisphosphonate therapy. Since toxicities are related to the frequency and total dosage, administration is limited to one dose, with additional dosing only if hypercalcaemia recurs.
- •
Glucocorticoids: prednisone 40–100 mg p.o. q.d. or hydrocortisone 200–300 mg i.v. for 3–5 days. Glucocorticoids are used primarily to augment the effect of calcitonin or in diseases associated with vitamin D excess (i.e. granulomatous diseases, vitamin D toxicity and multiple myeloma). Glucocorticoids increase calciuresis, decrease intestinal absorption of calcium and have a direct tumoricidal effect on certain haematological malignancies as well as breast cancer.
- •
Oral inorganic phosphate: phosphate 1–1.5 g p.o. q.d. Oral inorganic phosphate has a limited effect in normalising serum calcium in patients who are hypophosphataemic by increasing calcium uptake by bone and intestinal absorption of calcium. Intravenous phosphate is one of the swiftest means to reduce serum calcium levels. However, it can cause fatal hypocalcaemia and severe organ failure by calcium phosphate precipitation. As such, intravenous phosphate is reserved for life-threatening hypercalcaemia, and even then must be used with extreme caution.
- •
Dialysis. This is the treatment of choice for patients with hypercalcaemia and renal or heart failure. Dialysis may also be considered in hypercalcaemic patients who fail standard therapies. Haemodialysis and peritoneal dialysis can remove up to 250 mg of calcium/hour. Care must be taken to avoid the hypophosphataemia that often accompanies dialysis.
| Treatment | Onset | Duration | Effectiveness (% normalised) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| First-line therapy | |||||
| Normal saline | Hours | During use | 0–10 | Almost always dehydrated | Congestive heart failure Hypokalaemia/hypomagnesaemia |
| Loop diuretic | Hours | 2–6 hours | 0–10 | Fast onset | Electrolyte abnormalities Dehydration |
| Bisphosphonates | |||||
| Etidronate (first generation) | 1–2 days | 5–7 days | 30–80 | Intermediate onset | Hyperphosphataemia 3-day infusion |
| Pamidronate (second generation) | 1–2 days | 10–14 days | 70–100 | High potency | Fever (20%) |
| Prolonged duration | Hypophosphataemia/hypocalcaemia | ||||
| Intermediate onset | Hypomagnesaemia | ||||
| Calcitonin (salmon) | Hours | 2–3 days | 10–20 | Fast onset Bridge until intermediate-action drugs take effect | Tachyphylaxis Flushing Nausea/vomiting |
| Second-line therapy | |||||
| Plicamycin | 1–2 days | Days | 75–85 | High potency | Hepatocellular necrosis Bleeding (decreased clotting factors) Thrombocytopenia Renal failure Electrolyte abnormalities Hypocalcaemia |
| Gallium nitrate | Day 6 | 7–10 days | 75–82 | High potency | 5-day infusion Contraindicated in renal failure Hypophosphataemia Anaemia Nausea/vomiting Rare hypotension |
| Glucocorticoids | 5–7 days | Days to weeks | Variable | Oral therapy | Only effective in vitamin D excess or granulomatous disease |
| Cidal effect on haematological and breast cancers | Immunosuppression Cushing’s syndrome | ||||
| Phosphates | |||||
| Oral | 24 hours | During use | Variable | Low toxicity | Only effective in hyperphosphataemia |
| Intravenous | Hours | 1–2 days | Variable | High potency Rapid action | Severe hypocalcaemia Organ damage Potentially lethal |
The underlying cause of hypercalcaemic crisis must always be addressed as part of the definitive management. In patients with an elevated PTH level and clinical factors suggestive of PHP, parathyroidectomy is the fastest way to decrease PTH levels and consequently serum calcium levels. Therefore, expedient operative intervention should always be considered in this subgroup of patients.
In contrast, patients with malignancy-associated hypercalcaemic crisis typically present at advanced or terminal stages of their disease with a mean survival of only months. In this setting, discussion with the patient and family regarding end-of-life decisions will be appropriate.
Surgical indications
The treatment of PHP is primarily surgical as medical interventions do not address the underlying pathology. Medical treatment is generally temporary and is reserved for acute hypercalcaemic crises or for patients who have mild disease with low risk of long-term sequelae or are poor operative candidates based on age or comorbidities. Definitive therapy is focused on removal of the offending gland or glands. Box 1.3 lists the current indications for surgery in PHP, which include (1) symptoms, (2) age less than 50, (3) significant hypercalcaemia, (4) osteoporosis and (5) decreased renal filtration. Recommendations for parathyroidectomy in asymptomatic patients were updated in 2008, as surgical intervention decreases the long-term risks of hypercalcaemia on bone health and nephrolithiasis in broad patient populations.
- •
Symptoms
- •
Nephrolithiasis
- •
Age < 50
- •
Serum calcium > 1.0 mg/dL (0.25 mmol/L) above upper limit of normal
- •
Creatinine clearance reduced to < 60 mL/min
- •
Bone mineral density T-score < − 2.5 at any site and/or previous fracture fragility
- •
Patients requesting surgery or patients unsuitable for long-term surveillance
Imaging and localisation
In the hands of experienced surgeons, bilateral neck exploration for PHP cures 95% of cases. Furthermore, prior to recent advances in imaging technology, the sensitivity of localisation studies was approximately 60–70%.
Given these facts, the National Institutes of Health released guidelines in 1990 for the treatment of PHP that included the recommendation that preoperative localisation was not indicated.
Localisation studies were to be limited to re-operative cases. However, the advent of rapid intraoperative PTH assays and the highly sensitive and specific sestamibi scan (see below) have rekindled interest in preoperative localisation for directed unilateral exploration – the so-called focused approach.
Most patients with PHP have a single adenoma, while entities such as multiple adenoma and four-gland hyperplasia are considerably less frequent.
In a systematic literature review of 20 225 cases of PHP reported, Ruda et al. found that solitary adenomas, multiple gland hyperplasia, double adenomas and parathyroid carcinoma occurred in 88.9%, 5.74%, 4.14% and 0.74% of cases, respectively.
These statistics are consistent across the literature. The fact that the overwhelming majority of patients have unilateral disease or bilateral disease that can be identified by unilateral exploration raises the issue of whether bilateral exploration is mandated in every case. Is it reasonable to expose the patient to the increased morbidity of bilateral exploration to identify the less than 3% of people who will have a second adenoma on the contralateral side? These issues have led many endocrine surgeons to investigate the feasibility of preoperative localisation and directed unilateral exploration. This trend, along with the need to localise pathology in re-operative situations, has spurred the refinement of imaging techniques for parathyroid disease. Table 1.2 provides a summary of the current imaging modalities.
| Study | Sensitivity (%) | Specificity (%) | Re-operative sensitivity (%) | False positives (%) | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Ultrasound guidance | 71–80 | 80 | 40 | 15–20 | Inexpensive Fast Morphology No radiation/no i.v. contrast Confirms findings Can combine with fine-needle aspiration (FNA) | Difficulty with posterior areas and mediastinum Operator dependent Cannot detect lesions below 5 mm |
| Endoscopic ultrasound | 71 | – | – | – | Posterior/perioesophageal areas | Difficulty with anterior/lateral areas |
| CT scan with i.v. contrast | 46–80 | 80 | – | 50 | Mediastinum align Retro-oesophageal/ retrotracheal areas Can combine with FNA | Difficulty with lower neck around shoulders/thyroid area Previous surgery yields artefact Radiation Needs i.v. contrast |
| Magnetic resonance imaging | 64–88 | 88–95 | 50–88 | 18 | Localising ectopic glands Used if scintigraphy fails to localise lesion No i.v. contrast | Expensive Cannot be combined with FNA Compliance sometimes limited by claustrophobia Cannot detect lesions below 5 mm |
| Thallium–technetium scan | 75 | 73–82 | 50 | 25 | Wide availability Minimal radiation | Poor anatomical detail Average sensitivity |
| Technetium sestamibi scan | 90.7 | 98.8 | – | Low | Best localisation modality Minimal radiation Widely available SPECT offers excellent anatomical localisation Not operator dependent | May not identify four-gland hyperplasia or multiple adenomas |
| Angiography | – | – | 60 | – | Precise anatomical localisation | Neurological complications |
| Angiography and venous sampling | – | 96–98 | 91–95 | Low | Re-operative localisation | Embolisation Dye-induced renal failure |
| Venous sampling | – | – | 80 | 6–18 | Identifies multiple adenoma and four-gland hyperplasia |
Ultrasound (US)
Ultrasound was one of the first localisation techniques to be widely used. Typically this test is performed with the 7.5- or 10-MHz probes to optimise penetration and resolution. It is fast, non-invasive, non-irradiating and inexpensive. Furthermore, it allows visualisation of the thyroid, carotid, jugular and cervical areas. However, ultrasound is dependent on operator experience and size of pathology (limit is approximately 5 mm). This technique also has difficulty locating abnormalities in the retro-oesophageal, retrosternal, retrotracheal and deep cervical areas. False-positive results (15–20%) are due to muscles, vessels, thyroid nodules, lymphadenopathy and oesophageal pathology. Image quality may be limited by patient motion or metallic clips from previous operations. The reported sensitivity of ultrasound is between 71% and 80%, but falls to 40% for re-operative localisation.
Endoscopic US has also been used to evaluate posterior, deep cervical and perioesophageal glands. Endoscopic US correctly identified 12 of 23 adenomas (the remaining 11 were in either the anterior or lateral neck) in one series and had a sensitivity of 71% in another. Endoscopic US appears to have a role in localising certain parathyroid lesions for recurrent or persistent hyperparathyroidism.
Given these limitations, US is perhaps most useful when used in conjunction with other modalities. US combined with thyroid scintigraphy has the specific benefits of identifying intrathyroidal adenomas and distinguishing adenomas from thyroid nodules. Performing US-guided fine-needle aspiration (FNA) increases the sensitivity of US localisation by confirming the presence of PTH in the mass. Cytological studies of the aspirate are not useful and often cannot even distinguish between thyroid and parathyroid tissue. In one small series, PTH analysis of the aspirate made the diagnosis in 100% of cases. Finally, US provides a useful means to define the depth and singularity of adenomas found by scintigraphy.
Computed tomography (CT)
With the new-generation CT scanners and alterations in technique, the accuracy of CT has improved greatly over the last 5 years. In the past, the limitations of CT were based primarily on the size of the adenoma in that smaller parathyroid adenomas were more difficult to visualise. CT scan had difficulty in localising adenomas in the lower neck (at the level of the shoulders) and close to or within the thyroid. Furthermore, CT scan was inaccurate in differentiating between upper and lower pole glands. CT scans with intravenous contrast had sensitivities in the 80% range, but prior operations in the neck can produce artefacts, such as the ‘sparkler effect’ (seen with surgical clips), which reduce this number. The false-positive rate, at 50%, is higher than in other imaging modalities.
The accuracy of CT scanning is largely dependent on the technique utilised, as well as the experience and dedication of the radiologist interpreting the study. Whereas in the past most reports of CT scanning utilised 5-mm cross-sectional cuts, accurate parathyroid CT localisation mandates the use of 2.5-mm cuts as well as a dedicated radiologist committed to conducting the time-consuming review of parathyroid CT scans. Comparing pre- and post-intravenous contrast scans permits identification of parathyroid adenomas due to the increased vascularity of hyperfunctioning parathyroid tissue. Thin-cut parathyroid CT scanning provides precise anatamomical information regarding gland location (anterior, posterior, superior, inferior or mediastinum) as well as information regarding parathyroid gland relationship to the thyroid gland. Thyroid nodules can be differentiated from parathyroid adenomas due to the difference in shape and vascularity. Moreover, parathyroid gland weight can be estimated by determining the volume of the visualised parathyroid gland. As with US, CT may be used in conjunction with FNA to increase diagnostic yield. In a retrospective review from Columbia and Cornell Universities, Harari et al. demonstrated that in patients with negative sestamibi localisation, thin-cut CT scanning permitted a focused parathyroidectomy in 66% of patients. Four-dimensional reconstruction is now feasible and permits a remarkable appreciation and increased accuracy of parathyroid gland location and relationship to surrounding structures.
Magnetic resonance imaging (MRI)
MRI is superior to CT scanning in that it does not require intravenous contrast nor is it subject to the ‘sparkler effect’ or shoulder artefact. On T2-weighted imaging, enlarged parathyroid glands have significantly increased intensity. T2-weighted MRI is an excellent means of localising ectopic glands in patients undergoing re-operation for PHP, although it was less useful for identifying lesions in normal positions. Aufferman et al. found that MRI located 79% of ectopic adenomas while localising only 59% of those in the normal anatomical position. Overall sensitivities are in the 50–88% range for re-operative localisation. Despite better sensitivities (64–88%) than CT scanning, MRI has significant drawbacks. This modality cannot image normal glands or adenomas less than 5 mm in size. Furthermore, it has difficulty localising superior parathyroid glands since they lie posterior to the thyroid. False positives can result from thyroid nodules and lymphadenopathy. Finally, MRI is expensive, cannot be combined with FNA, and patient compliance is sometimes limited by claustrophobia. Given all these factors, MRI is best reserved for localisation in re-operation for PHP or when parathyroid scintigraphy is negative or equivocal.
Thallium-201–technetium-99 m pertechnetate scan (Tl– 99m Tc scan)
Tl–Tc scanning is an image subtraction technique that is rapidly being replaced by sestamibi scanning (see below). Tl–Tc scanning relies on the fact that the thyroid and parathyroid tissues (especially hyperfunctioning glands) take up thallium while the thyroid alone takes up technetium. By subtracting the two images, one can localise the parathyroid tumour. The sensitivity of Tl–Tc scanning is 75% for first-time operations and only 50% for re-operations. The false-positive rate is approximately 25% and occurs with metastatic nodal disease and thyroid pathology. Given the average sensitivity and poor anatomical detail of Tl–Tc scanning, this mode has been relegated to second-line imaging status.
Technetium-99 m sestamibi scan (sestamibi scan)
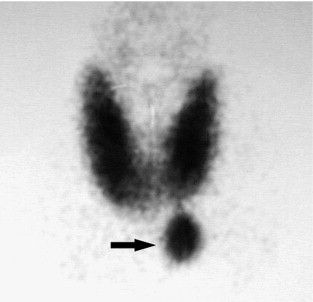
Ever since Coakley et al. fortuitously discovered that technetium-99 m sestamibi concentrated in abnormal parathyroid glands, sestamibi scanning has revolutionised the practice of parathyroid surgery, making directed unilateral exploration a reasonable alternative to routine bilateral exploration. Sestamibi is a derivative of technetium that avidly incorporates itself into mitochondria. The large amount of mitochondria in hyperactive parathyroid glands allows more intense labelling of parathyroid tumours relative to the thyroid and surrounding tissue. The radiotracer also washes out much more slowly from the parathyroid than the thyroid. This differential uptake can be accentuated by pretest medical thyroid suppression. Sestamibi exploits these differences in uptake and retention to localise parathyroid adenomas. This radioisotope has a short half-life and produces high-energy photon emission that allows for low doses of radiation and high-definition imaging. Also, sestamibi scanning images both in the anteroposterior and lateral views, which allows for more precise localisation of the pathology.
There are three basic protocols for sestamibi scanning in current use:
- •
Single-isotope dual-phase scan. After intravenous administration of 15–25 mCi of sestamibi, images are taken at 10, 15, 120 and 180 minutes post-injection. A positive scan demonstrates increased uptake of tracer in the thyroid gland and parathyroid adenoma in early phases with washout of tracer from the thyroid gland but not the parathyroid adenoma in the late-phase images. This is the simplest and most widely used protocol. However, two potential pitfalls of this technique are: (i) sestamibi can accumulate and remain in thyroid nodules; and (ii) rapid washout of sestamibi can lead to false-negative results. To counter the first problem, many investigators are experimenting with dual-isotope subtraction scanning. Figure 1.1 illustrates a typical parathyroid adenoma in the early-phase scan.