Introduction
Pancreatic neuroendocrine tumours (pNETs) are the most common endocrine tumours found in the abdomen. They may be broadly classified into two groups: functional tumours, which cause clinical syndromes due to hormone secretion; and non-functional tumours, which may cause symptoms through mass effect or metastatic spread, or may be discovered incidentally. The most common functional pNETs are gastrinomas and insulinomas, but a variety of other rare hormone secreting tumours also occur ( Table 5.1 ). Identification of clinical syndromes and hormone production allows the classification of functional pNETs into specific types. Potentially life-threatening situations caused by hormone overproduction are a major reason to identify and resect these neoplasms. Additionally, pNETs have malignant potential. However, they are less malignant than pancreatic adenocarcinoma and surgical extirpation is beneficial in most instances.
| Location (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tumour | Incidence (people per million per year) | Hormone secreted | Signs or symptoms | Diagnosis | Duodenum | Pancreas | Malignant (%) | MEN1 (%) |
| Gastrinoma | 0.1–3 | Gastrin | Ulcer pain diarrhoea, oesophagitis | Fasting serum gastrin > 100 pg/mL Basal acid output > 15 mEq/h | 38 | 62 | 60–90 | 20 |
| Insulinoma | 1 | Insulin | Hypoglycaemia | Standard fasting test | 0 | > 99 | 5 | 5–10 |
| VIPoma | Vasoactive intestinal peptide (VIP) | Watery diarrhoea, hypokalaemia, hypochlorhydria | Fasting plasma VIP > 250 pg/h | 15 | 85 | 60 | < 5 | |
| Glucagonoma | Glucagon | Rash, weight loss, malnutrition, diabetes | Fasting plasma glucagon > 500 pg/h | 0 | > 99 | 70 | < 5 | |
| Somatostatinoma | Somatostatin | Diabetes, cholelithiasis, steatorrhoea | Increased fasting plasma somatostatin concentration | 50 | 50 | 70 | < 5 | |
| GRFoma | 0.2 | Growth hormone-releasing factor (GRF) | Acromegaly | Increased fasting plasma GRF concentration | 0 | 100 | 30 | 30 |
| ACTHoma | Adrenocorticotropic hormone (ACTH) | Cushing’s syndrome | 24-hour urinary free cortisol > 100 μg, plasma ACTH > 50 pg/h no dexamethasone suppression, no CRH suppression | 0 | 100 | 100 | < 5 | |
| PTH-like-oma | Parathyroid hormone (PTH)-like factor | Hypercalcaemia, bone pain | Serum calcium > 11 mg/dL, serum PTH undetectable, increased serum PTH-like factor | 0 | 100 | 100 | < 5 | |
| Neurotensinoma | Neurotensin | Tachycardia, hypotension, hypokalaemia | Increased fasting plasma neurotensin concentration | 0 | 100 | > 80 | < 5 | |
| Calcitonin-secreting pNET | Calcitonin | Diarrhoea | Rare | |||||
| Non-functioning (PPoma) | 1–2 | Pancreatic polypeptide (PP), chromogranin A, neuron-specific enolase | Pain, bleeding mass | Increased plasma concentration of PP, chromogranin A or neuron-specific enolase | 0 | > 99 | > 60 | 80–100 |
Insulinoma
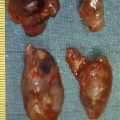
Endogenous hyperinsulinism was first described in 1927, and was the first syndrome of excessive pancreatic hormone production to be recognised. Hyperinsulinaemia and consequent hypoglycaemia is the major cause of morbidity and potential mortality associated with insulinoma. Insulinoma occurs in approximately one person per million population per year ( Table 5.1 ). Hyperinsulinaemic hypoglycaemia associated with insulinoma is not well controlled by medical therapy, and surgery has remained the cornerstone of treatment over the past 80 years. Insulinomas are unique among pNETs in that 90% of insulinomas are benign, solitary growths that occur uniformly throughout and almost exclusively within the pancreas, without evidence of local invasion or locoregional lymph node metastases. Tumours may be as small as 6 mm in diameter and are usually less than 2 cm in size, making localisation challenging in many cases.
Presentation
Excessive and physiologically uncontrolled secretion of insulin by the tumour causes episodes of symptomatic hypoglycaemia and leads patients to seek medical evaluation. Acute neuroglycopenia induces anxiety, dizziness, obtundation, confusion, unconsciousness, personality changes and seizures. Symptoms commonly occur during early morning hours, when glucose reserves are low after a period of overnight fasting during which insulin overproduction has continued. Most patients (80%) experience major weight gain as they attempt to treat these symptoms with increased caloric intake. They may first present with symptoms of hypoglycaemia when food intake is then decreased in an attempt to lose weight. A majority (60–75%) of patients are women, and many have undergone extensive psychiatric evaluation before the correct diagnosis is reached. Other patients will have been misdiagnosed with neurological conditions such as seizure disorders, cerebrovascular accidents or transient ischaemic attacks. Potentially life-threatening symptoms may be present for several years prior to diagnosis. In a review of 59 patients with insulinoma, the interval from onset of symptoms to time of diagnosis ranged from 1 month to 30 years, with the median time to diagnosis being 2 years. Because insulinoma is rare and neuroglycopenic symptoms are relatively non-specific, a high index of suspicion for insulinoma is necessary when other explanations for these symptoms are not evident. The identification of symptomatic patients and the liberal use of simple and precise biochemical tests result in accurate diagnosis of insulinoma prior to life-threatening sequelae.
Diagnosis
The diagnosis of insulinoma is established through a 72-hour supervised fast. The most definitive biochemical result for insulinoma is a plasma insulin concentration above 5 μU/mL in the presence of symptomatic hypoglycaemia. Factitious hypoglycaemia must be excluded.
The classic diagnostic triad, proposed by Whipple in 1935 based on his observations in 32 patients, consists of symptoms of hypoglycaemia during a fast, a concomitant blood glucose concentration less than 3 mmol/L and relief of the hypoglycaemic symptoms after glucose administration. Factitious hypoglycaemia due to clandestine administration of exogenous insulin or oral hypoglycaemic drugs may also result in this same presentation and can lead to a misdiagnosis of insulinoma. Factitious hypoglycaemia classically occurs in patients associated with the medical profession or patients who have relatives with diabetes. The diagnosis of insulinoma must be reached in each patient by performing a 72-hour supervised fast with appropriate biochemical measurements. Urinary sulphonylurea concentrations (to exclude oral hypoglycaemic drugs) should be measured by gas chromatography–mass spectroscopy.
Supervised standard fasting test
The standard fasting test is carried out in a hospital setting and begins with a baseline examination in which memory, calculations and coordination are documented ( Fig. 5.1 ). An intravenous catheter with a heparin lock is then placed, and the patient is allowed to drink only non-caloric beverages. Close observation is necessary. Blood is collected every 6 hours for measurement of serum glucose and immunoreactive insulin concentrations. As the blood glucose level falls below 3 mmol/L, blood samples are collected more frequently (every hour or less) and the patient is observed more closely. When neuroglycopenic symptoms appear, blood is collected immediately for determination of serum insulin, glucose, C-peptide and proinsulin concentrations ( Fig. 5.1 ). Glucose is then administered and the fast is terminated. If a patient remains symptom-free for the entire 72 hours, the test is terminated and the above blood concentrations are measured.

Neuroglycopenic symptoms manifest in approximately 60% of patients with insulinomas within 24 hours after fasting begins. Approximately 16% of patients with insulinoma develop symptoms when the blood glucose concentration is greater than 2.5 mmol/L. The blood glucose concentration eventually decreases below 2.5 mmol/L in approximately 85% of patients with insulinomas during the 72-hour fast ( Table 5.2 ). The most definitive diagnostic biochemical test for insulinoma is an inappropriately increased plasma immunoreactive insulin concentration above 5 μU/mL at the time of documented hypoglycaemia and symptoms. The plasma insulin concentration is usually greater than 10 μU/mL. Although prolonged maximal stimulation of insulin secretion in normal subjects does not cause the release of the insulin precursor molecule proinsulin, some insulinomas secrete large amounts of uncleaved proinsulin. Patients with high proinsulin-producing tumours may remain euglycaemic and asymptomatic for longer periods during the fast because proinsulin is not biologically active. The proinsulin-like component (PLC) is measured at the time of symptomatic hypoglycaemia and termination of the fast. A value greater than 25% or an increased PLC: total immunoreactive insulin ratio is abnormal and consistent with the diagnosis of insulinoma. Hypersecretion of endogenous insulin also results in increases of the circulating concentration of C-peptide, a biologically inactive by-product of enzymatic insulin cleavage from the precursor proinsulin molecule. Most patients with insulinomas have C-peptide concentrations greater than 1.7 ng/mL.
| Blood measurement | Fasting normal range | Result with insulinoma | Result with factitious hypoglycaemia | Test sensitivity (%) |
|---|---|---|---|---|
| Glucose | 90–150 mg/dL | < 40 mg/dL | < 40 mg/dL | 99 |
| Immunoreactive insulin (IRI) | < 5 μU/mL | Increased | Increased (usually > 10 μU/mL) | 100 |
| C-peptide | < 1.7 ng/mL | Increased | Normal range | 78 |
| Direct proinsulin-like component (PLC) | < 0.2 ng/mL | Increased | Normal range | 85 |
| PLC/total IRI | < 25% | Increased | Normal range | 87 |
Increased serum concentrations of proinsulin or C-peptide during hypoglycaemia effectively exclude the diagnosis of factitious hypoglycaemia because exogenously administered insulin does not contain these proteins and actually suppresses their production. However, approximately 13–22% of patients with insulinoma do not have increased serum proinsulin or C-peptide concentrations, and a supervised fast prohibiting exogenous insulin administration remains the best test to diagnose insulinoma and conclusively exclude factitious hypoglycaemia. The biochemical parameters measured during the standard fasting test cannot discriminate between patients with MEN1 and patients with sporadic insulinoma.
Nesidioblastosis
Insulinoma must be distinguished from nesidioblastosis, a condition of islet cell hyperfunction and malregulation that occurs primarily in infants and causes hyperinsulinaemic hypoglycaemia. Age at the time of presentation is the most important distinguishing factor, as nesidioblastosis occurs most commonly in children under the age of 18 months. Approximately half of infants with nesidioblastosis require a spleen-preserving near-total pancreatectomy, in which 95% of the pancreas is removed, because this disorder affects the entire pancreas diffusely.
Adult nesidioblastosis has been reported and was recently described in a small series of adult patients following gastric bypass surgery for morbid obesity. However, nesidioblastosis in adults is exceedingly rare. In a review of over 300 patients with hyperinsulinaemic hypoglycaemia evaluated at the Mayo Clinic since 1927, only five adult patients had a reasonably confirmed diagnosis of nesidioblastosis. Furthermore, biochemical tests (blood glucose, insulin and C-peptide) do not reliably distinguish hyperinsulinaemic hypoglycaemia caused by an insulinoma from that attributed to nesidioblastosis, and an insulinoma may be present in a patient who has islet cell hyperplasia. Therefore, the diagnosis of nesidioblastosis in the adult must be critically suspect and should not preclude attempts to localise an insulinoma.
Management
Medical management of hypoglycaemia
Medical management aims to prevent hypoglycaemia caused by hyperinsulinism so that symptoms and life-threatening sequelae are avoided. In patients with acute hypoglycaemia, blood glucose concentrations are normalised initially with an intravenous dextrose infusion. To prevent hypoglycaemic episodes during diagnosis, tumour localisation and the preoperative period, euglycaemia is maintained by giving frequent feeds of a high-carbohydrate diet, including a night feed. For patients who continue to become hypoglycaemic between feedings, diazoxide may be added to the treatment regimen at a dose of 400–600 mg orally each day. Diazoxide inhibits insulin release in approximately 50% of patients with insulinoma; however, side-effects of oedema, weight gain and hirsutism occur in 50% of patients, and nausea occurs in over 10%. Diazoxide should be discontinued 1 week prior to surgery as it may contribute to intraoperative hypotension. Calcium-channel blockers or phenytoin may also suppress insulin production in some patients. Long-term control of hypoglycaemic symptoms with medical management has generally been ineffective for patients with insulinoma. The surgeon must know the patient’s response to medical management in order to gauge the urgency of surgical intervention.
Octreotide, a synthetic, long-acting analogue of the naturally occurring hormone somatostatin, may be useful for treating symptoms caused by vasoactive intestinal peptide (VIP)-omas and carcinoid tumours but is not generally recommended for insulinomas because its efficacy in inhibiting insulin release is unpredictable. The usefulness of radiolabelled octreotide in imaging insulinomas has been equally disappointing. Therefore, long-term medical management of hypoglycaemia in patients with insulinomas generally is reserved for the few patients (< 5%) with unlocalised, unresected tumours after thorough preoperative testing and exploratory laparotomy, and for patients with metastatic, unresectable malignant insulinoma. Patients with malignant insulinomas and refractory hypoglycaemia may even require the placement of implantable glucose pumps for continuous glucose infusion.
Preoperative tumour localisation
Virtually all insulinomas may be localised by the experienced surgeon through the combination of preoperative modalities and intraoperative ultrasound. Blind pancreatic resection is not indicated.
After definitive biochemical diagnosis, the tumour must be localised and the presence of unresectable metastatic disease excluded. Accurate tumour localisation is often the most difficult aspect of management because insulinomas are usually small and solitary.
Non-invasive imaging studies
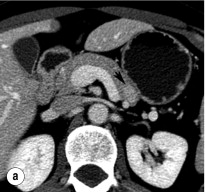
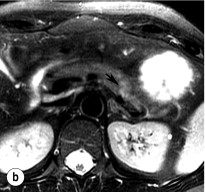
An initial attempt should be made to localise the tumour and identify metastatic disease by using non-invasive tests. Computed tomography (CT) and magnetic resonance imaging (MRI) are both capable of identifying pancreatic tumours as small as 1 cm in diameter ( Table 5.3 ). If CT is elected, a pancreatic protocol study with biphasic contrast injection and fine cuts through the pancreas should be used. Tumours will appear hypervascular on arterial phase images ( Fig. 5.2 a ). The sensitivity of CT for insulinoma is 40–80% in recent series. MRI may image an islet cell tumour based on increased signal intensity (brightness) on T2-weighted images ( Fig. 5.2b ). The sensitivity of MRI is similar to that of CT and, as expected, the accuracy of both CT and MRI increases with larger tumour size.
| % of tumours localised | |||||
|---|---|---|---|---|---|
| Gastrinoma | |||||
| Study | Insulinoma | Overall | Pancreas | Duodenum | Liver metastases |
| Preoperative | |||||
| Non-invasive | |||||
| Abdominal CT | 40–80 | 50 | 80 | 35 | 50 |
| Abdominal MRI | 11–43 | 25 | 83 | ||
| Octreoscan | 0–50 | 88 | |||
| Invasive | |||||
| Endoscopic ultrasonography | 70–90 | 85 | 88–100 | < 5 | < 5 |
| Selective arteriography | 40–70 | 68 | 34 | 86 | |
| + calcium stimulation | 88–94 | – | – | – | – |
| + secretin injection | – | 90–100 | |||
| Unlocalised primary tumour | 10–20 | 15 | |||
| Intraoperative | |||||
| Palpation | 65 | 65 | 91 | 60 | |
| Intraoperative ultrasonography | 75–100 | 83 | 95 | 58 | |
| Duodenotomy | – | – | – | 100 | |
| Unlocalised primary tumour | 1 | 5 | |||
The rare malignant insulinoma is almost always very large (> 4 cm) and easily imaged by CT or MRI. Bulky metastatic tumour deposits and hepatic metastases are also usually readily identifiable by CT or MRI. Metastases should be identified preoperatively so that the operative approach can be planned or, in the case of unresectability, unnecessary surgery avoided.
Invasive localising procedures
Up to 50% of insulinomas are too small to be detected by non-invasive imaging. A variety of more sensitive invasive tests are used to localise these tumours preoperatively. Endoscopic ultrasound (EUS) is safe and highly effective in experienced hands and is the modality of choice for patients whose insulinoma is not localised by CT. It has been demonstrated to detect tumours as small as 2–3 mm, well below the limits of detection of CT or MRI, and it is the most sensitive modality for detection of intrapancreatic tumours.
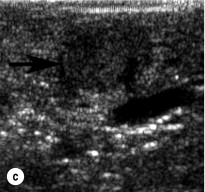
To perform EUS of the pancreas, the endoscope is passed into the duodenum and a saline-filled balloon is inflated against the intestinal wall. A 5- to 10-MHz transducer is used to generate an image of the pancreas through the intestinal and stomach walls. Tumours as small as 2–3 mm in diameter can be identified at the pancreatic head by moving the transducer through the duodenum at the junction with the pancreas. The endoscope must be passed well into the third portion of the duodenum to adequately visualise the uncinate process. Insulinomas in the pancreatic body and tail are imaged by positioning the transducer in the stomach and scanning through its posterior wall. Insulinomas will appear homogeneously hypoechoic, well circumscribed and round, and are typically easily distinguishable from the surrounding pancreatic parenchyma. Sensitivity of EUS for insulinoma ranges from 70% to 94%. Rates of detection are highest in the head of the pancreas (83–100%) because the head can be viewed from three angles (from the third portion of the duodenum, through the bulb of the duodenum and through the stomach). They are lower (37–60%) in the body and tail, which can only be viewed through the stomach.
Despite the tremendous potential and proven benefit of EUS for insulinoma, there are some limitations. First, there may be false positives, which include accessory spleens and intrapancreatic lymph nodes. Further, EUS is limited in assessment of malignancy, identification of pedunculated tumours, and differentiation of large tumours from the pancreatic parenchyma.
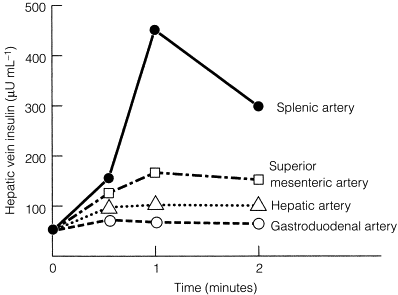
In patients with negative results after non-invasive imaging studies and EUS, calcium arteriography may be useful ( Fig. 5.3 ). This study relies on the functional activity of the insulinoma (i.e. excessive insulin production) and not on the ability to image the tumour (i.e. tumour size). Arteries that perfuse the pancreatic head (gastroduodenal artery and superior mesenteric artery) and the body/tail (splenic artery) are selectively catheterised, and a small amount of calcium gluconate (0.025 mEq Ca 2 + /kg body weight) is injected into each artery during sequential runs. A catheter positioned in the right hepatic vein is then used to collect blood for measurement of insulin concentrations. Calcium stimulates a marked increase in insulin secretion from the insulinoma. A greater than twofold increase in the hepatic vein insulin concentration indicates localisation of the tumour to the area of the pancreas being perfused by the injected artery ( Fig. 5.3 ). In this way, calcium provocation may identify the region of the pancreas containing the tumour (head, body or tail). Additionally, injection of contrast may reveal a tumour blush, confirming the location of the insulinoma by imaging the tumour. These combined features are particularly useful in identifying the insulinoma in patients with MEN1 who may have multiple imaged pNETs. The reported sensitivity of calcium stimulation is between 88% and 94% and few false-positive results occur ( Table 5.3 ).
A small proportion of insulinomas will remain unlocalised even after all preoperative studies are obtained. When the diagnosis is certain on the basis of the results of the supervised fast, surgical exploration with careful inspection, palpation and intraoperative ultrasonography (IOUS) of the pancreas is still indicated. Most of these patients (> 90%) will still have an insulinoma identified and removed by the experienced surgeon. Retrospective reviews have shown that the combination of careful surgical exploration with IOUS will identify almost all insulinomas.
Operative management
Open exploration
In contradistinction to gastrinomas, virtually all insulinomas are located within the pancreas and are uniformly distributed throughout the entire gland. Therefore, in the patient with unlocalised insulinoma, the head, body and tail of the pancreas should be sufficiently mobilised to permit evaluation of the entire organ. This requires an extended Kocher manoeuvre, to adequately lift the head of the pancreas out of the retroperitoneum, and division of attachments at the inferior and posterior border of the pancreas, to permit evaluation of the posterior body and tail ( Fig. 5.4 ). The entire pancreatic surface should then be inspected, as an insulinoma may appear as a brownish-red purple mass. Because the head of the pancreas is thick, small tumours that are centrally located may not be easily palpated. The entire pancreatic head must be sufficiently mobilised so that the posterior surface can be adequately examined visually and palpated between the thumb and forefinger. The splenic ligaments may be divided to completely mobilise the spleen out of the retroperitoneum for full examination and palpation of the pancreatic tail.