Pancreatic ductal adenocarcinoma (PDA) is the fourth leading cause of cancer-related deaths in the United States and the eighth worldwide. Despite advances in medical therapy, survival remains poor. The median survival for patients diagnosed with PDA is 4 to 6 months. However, for the 10% to 20% of patients who are operative candidates at the time of diagnosis, the 5-year overall survival approaches 25%, and the median survival is in the range of 20 to 22 months. The incidence of pancreatic cancer has slowly risen over the last decade, resulting in over 310,000 annual deaths worldwide.1 The aggressive nature of pancreatic cancer can be seen as each year the incidence nearly matches the death rate, and accounts for about 42,000 deaths annually in the United States alone.2 Given this aggressiveness and poor long-term outcomes, high variability exists in the surgical approach to patients with pancreatic cancer. Many aspects of surgery for pancreatic cancer have been evaluated including the appropriate workup and staging, need for laparoscopy, need for extended lymphadenectomies, and the use of vascular reconstruction to improve morbidity and survival. The following is a comprehensive review of the available data regarding the surgical management of patients with resectable pancreatic cancer.
Pancreatic cancer often presents late in its disease course with various nonspecific symptoms making early diagnosis difficult. Many patients do not typically present with symptoms until their disease has progressed, often past the point where surgical therapy can offer a cure. Patients with tumors located in the body and tail usually present after the tumor grows sufficiently large to cause invasion of contiguous structures and/or metastatic disease. Most patients with PDA present with lesions in the pancreatic head or neck (65%), with 15% present in the body/tail, and the remaining 20% being diffuse in nature. The classically described “Courvoisier’s sign” is present only in 30% of people at the time of diagnosis. Patients often present with symptoms including abdominal pain (70%), fatigue (60%), malaise (60%), and painless jaundice (50%). Other presenting symptoms may include clay-colored stool, dark urine, pruritus, weight loss, and anorexia. New-onset diabetes can be a sign of pancreatic cancer, with up to 30% of newly diagnosed patients receiving a diagnosis of diabetes within the preceding 2 years.3,4
The staging of tumors of the pancreas continues to evolve and most recently follows the American Joint Committee on Cancer (AJCC), seventh edition, TNM staging system5 (Table 141-1). In the current version it should be noted that T3 tumors are those extending beyond the pancreas and not involving the superior mesenteric artery (SMA) or celiac axis. T3 disease no longer precludes patients from resection as venous reconstruction in these patients has survival rates similar to those without venous invasion. In addition, regional lymph node disease is categorized as being present or absent, without designation based upon number of nodes involved as in previous versions of the AJCC staging system. The current version emphasizes preoperative imaging in order to stratify patients who have invasion of the celiac axis or SMA and therefore unable to undergo local curative resection. The seventh edition attempts to identify patients with metastatic disease (stage IV) and local unresectable disease (stage III) versus those who are potentially resectable (stage I-IIB) based on high-resolution imaging (see Table 141-1).
TNM Classification of Pancreatic Ductal Adenocarcinomaa
| Tumor (T) | T0—No Evidence of Primary Tumor | Tis—In Situ | T1—Limited to Pancreas (<2 cm) | T2—Limited to Pancreas (>2 cm) | T3—Extends Beyond Pancreas Without Celiac Axis or SMA Involvement | T4—Involves Celiac Axis or SMA |
|---|---|---|---|---|---|---|
| Regional Lymph Nodes (N) | N0: No nodal metastasis | N1: Regional nodal metastasis | ||||
| Distant Metastasis (M) | Mx: Metastasis cannot be assessed | M0: No distant metastasis | M1: Distant metastasis | |||
| AJCC Stage | Stage 0: Tis, N0,M0 | Stage IA: T1, N0, M0 | Stage IIA: T3, N0, M0 | Stage III: T4, N0, M0 | Stage IV: T1-4, N0-1, M1 | |
| Stage IB: T2, N0, M0 | Stage IIB: T1-3, N1, M0 |
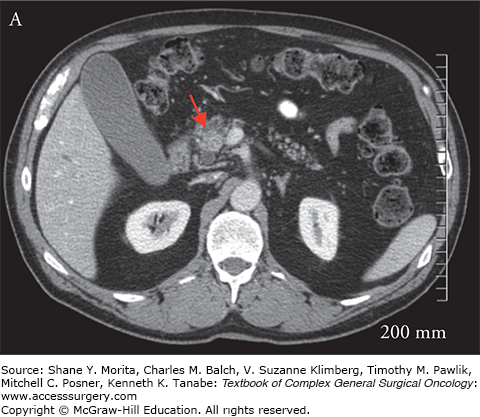
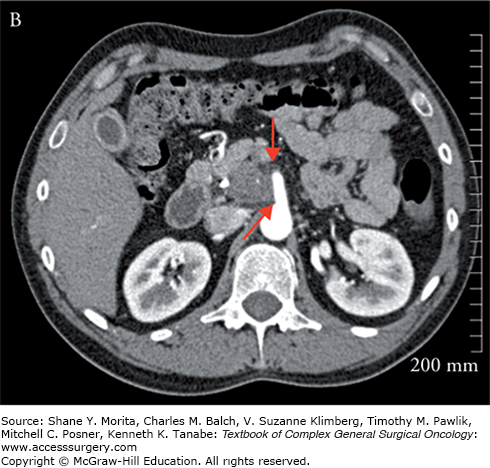
Once the diagnosis of pancreatic cancer is established, the best current treatment strategy is for complete resection of the primary tumor and regional lymph nodes. For complete resection to be achieved, preoperative imaging is key to assess for resectability, as patients who are not resectable achieve no benefit from aggressive surgical resection. The current accepted standard for those who should go forth with a surgical resection must meet the following criteria: (1) no distant metastasis; (2) absence of arterial involvement as determined by a visible fat plane between critical arterial structures, namely the superior mesenteric artery, celiac axis, and hepatic arteries; (3) less than 180 degree involvement of the superior mesenteric vein (SMV) or portal vein (PV); and (4) a patent PV/mesenteric vein confluence (Fig. 141-1A).6–8 Controversy exists in the optimal management of patients who meet some but not all of these criteria and are considered borderline resectable. Borderline resectable tumors are those that are technically resectable at presentation but carry a high risk of margin positivity if surgery is performed up-front. Two major classifications exist, providing specific criteria for borderline resectability, one from the MD Anderson (MDA) group and the other from the Americas Hepato-Pancreato-Biliary Association (AHPBA)/Society for Surgery of the Alimentary Tract9–11 (Fig. 141-1B). Both postulate that abutment of the SMA (<180 degree involvement) qualify as borderline resectable, but differ in that the MDA group considers minimal venous involvement resectable, with >180 degree involvement or complete venous occlusion meeting borderline resectability criteria. The AHPBA considers any degree of venous involvement meeting borderline resectability. More recently the Intergroup has revised the definition of “borderline resectable” to avoid the use of subjective terminology such as “abutment” or “impingement.” In their definition, tumors that are borderline resectable include those which meet one of the following: (1) any interface between the primary tumor and SMV-PV measuring 180° or greater the circumference of the vein wall; (2) short-segment occlusion of the vein, with normal vein proximal and distal to the occlusion that is amenable to resection and venous reconstruction; (3) short-segment interface of any degree of the hepatic arteries that are amenable to reconstruction; (4) an interface between the tumor and the SMA or celiac trunk measuring less than 180°.12 It is recommended that any tumor meeting borderline criteria be considered for downstaging with a multidisciplinary neoadjuvant strategy.
Preoperative imaging is a critical factor in the workup of patients with PDA. Imaging is necessary to define the relationship of the tumor to surrounding anatomic structures, determine resectability, and rule out metastatic disease. Multislice computed tomography (CT) scan with pancreatic protocol-specific contrast timing remains the accepted gold standard in the management of patients with PDA. For lesions >15 mm, a thin slice CT has a sensitivity and specificity of 100%.13 These high results are dependent on imaging obtained with proper technique. These include using oral water as a negative intraluminal contrast to allow for duodenal distension (150 mL). Additionally, intravenous contrast is administered at a rate of 3 to 4 mL/s with scanning during pancreas perfusion. Finally, a second-phase scan obtained at 60 to 70 seconds allows for optimal visualization of the liver parenchyma. This affords differentiation between normal hepatic parenchyma and hypovascular metastasis.14 Despite the advances in image resolution, up to 11% of pancreatic cancers can be isoattenuated on the pancreatic and hepatic phases, making identification difficult in the absence of other findings such as mass effect or ductal dilatation.15
Suspicious CT findings include loss of normal pancreatic glandular architecture, loss of peripancreatic fat planes, pancreatic parenchymal atrophy with ductal dilation, and encasement or involvement of surrounding structures including the celiac axis, SMA/SMV, or PV. Typically, pancreatic adenocarcinoma appears hypoattenuated compared to normal pancreatic parenchyma, and is usually best seen on venous phase of biphasic CT scan. Isolated glandular enlargement is also suggestive of a parenchymal mass; however, 20% of patients with pancreatic head masses have normal size glands with distal atrophy. Diffuse enlargement of the gland is more consistent with pancreatitis. Hypoattenuated masses may be homogenous or contain some fatty interstices. Additionally, areas of central necrosis can be seen. If CT is unclear, an endoscopic ultrasound (EUS) or CT-guided biopsy can be obtained. Solid hypodense masses do not need to undergo any further investigation, as they are most commonly abnormal, but a fine-needle aspiration (FNA) of a heterogeneous cystic structure can help to delineate pancreatic pseudocyst, organized pancreatic necrosis, or a tumor with a necrotic central zone, and benign cystic lesions.
While CT scans are universally accepted due to their relative availability, high resolution, and speed of imaging, the advancements in magnetic resonance imaging (MRI) in the recent years is continuing to evolve. MRI can help detect smaller lesions, including those <2 cm. This involves breath-holding techniques and can be enhanced with the administration of gadolinium contrast. Fat-suppressed spin-echo and single breath-hold gradient-echo fast low-angle shot (FLASH) sequences are the most sensitive modality to detect tumors compared to any other technique.16 Newer data are showing promise for diffusion-weighted MRI to help delineate between autoimmune pancreatitis (AIP) and malignancy. In a study of approximately 60 patients, Kamisawa et al17 determined that apparent diffusion coefficients (ADC) were significantly lower in AIP, and using a cutoff value of 1.075 × 103 mm2 resulted in optimal imaging distinction. The overall accuracy of MRI to establish a diagnosis approaches that of multislice CT scan, and is nearly 85% in the face of main pancreatic duct dilation; however, it is a much longer test that limits its quality if there is motion artifact. When combined with MR cholangiopancreatography, diffusion-weighted MRI allows for the detection of occult liver metastasis without the additional risk of an iodinated contrast medium.18
Metastasis is present in 17% to 55% of patients and is most commonly to the liver. This appears as hypodense lesions in the liver parenchyma. Additionally, lymph node enlargement can be seen and has an incidence as high as 65%.
When patients present with obstructive jaundice, in addition to imaging, endoscopic retrograde cholangiopancreatography (ERCP) should be considered as the next step to allow for biliary decompression. This is especially true for patients being considered for neoadjuvant therapy or those that present with unresectable disease. For patients with resectable disease, it is our practice to forgo biliary decompression if surgery can be performed in an expedited manner. The addition of EUS increases the sensitivity to detect lesions <15 mm and approaches 100%; however, visualization of smaller lesions is highly operator dependent. EUS also allows for US-guided FNA, with sensitivities and specificities comparable to that of CT guidance. Magnetic resonance cholangiopancreatography (MRCP) allows for visualization of the ductal structures with similar accuracy as with ERCP, but without the invasiveness of upper endoscopy, and avoids instrumentation of the biliopancreatic tree if not needed.
Since most pancreatic cancers are located in the pancreatic head, obstructive jaundice is often a common presenting symptom. Therefore, preoperative biliary drainage is often employed in order to alleviate the symptoms of pruritus or in the setting of neoadjuvant therapy in which surgical treatment will be delayed. Previously, the use of preoperative biliary diversion was routinely performed due to concerns about perioperative morbidity in a jaundiced individual undergoing a pancreaticoduodenectomy (PD).19,20 However, these studies are faulted by their retrospective nature and heterogeneous patient population. Modern literature does not corroborate these studies and recent randomized trials have proven these concerns unfounded.
Povoski et al21 showed that in 240 patients who underwent PD at Memorial Sloan Kettering Cancer Center in a 3-year period, 73% (175 patients) underwent preoperative biliary instrumentation, defined by cannulation of the biliary tract by various techniques. Of these, 126 patients subsequently received a drainage procedure via percutaneous, endoscopic, or surgical drainage modalities. Overall morbidity following PD was 48% with a mortality rate of 5% (12 of 240 patients). The only statistically significant factor associated with the overall complication rate was biliary stenting, causing an increased risk of overall complications (p = 0.025), infectious complications (p = 0.014), intra-abdominal abscess formation (p = 0.022), and postoperative death (p = 0.037).
In contrast to this study, Sohn et al22 from Johns Hopkins failed to demonstrate an increase in the overall complication rates in patients undergoing preoperative biliary drainage compared to those who did not. They retrospectively assessed 567 patients undergoing PD, with 72% (408 patients) receiving preoperative biliary stenting. Forty-seven percent of these patients undergoing PD specifically for PDA received stenting while 38% did not. When comparing the two groups of stented and unstented individuals, the only statistically significant difference was in that of rates of wound infection and pancreatic fistula formation (p = 0.02).
Pisters et al23 similarly examined their experiences from the MD Anderson Cancer Center, with 172 patients (57%) with malignancy or suspected malignancy receiving preoperative biliary drainage. Similar to previous studies, the majority of these drainage procedures consisted of endoscopic stents. Consistent with the aforementioned studies, they demonstrated an increased wound infection rate (p = 0.028) with 13% versus 4% in those that were stented or unstented, respectively.
In a large randomized, prospective, multi-institutional trial, van der Gaag et al24 aimed to assess whether early surgery versus preoperative drainage had differences in outcomes. Of 202 total patients enrolled, 106 patients were randomized to biliary drainage via endoscopically placed stent versus 96 randomized to early surgery. Despite no differences in mortality, or hospital stay, they did demonstrate an increase in overall complications in the drainage group (p < 0.001). Several other studies, however, have failed to recapitulate the data above, and thus a definitive consensus on preoperative biliary drainage has not yet been reached. Until that time, most recommend reserving preoperative biliary drainage for those patients who cannot undergo early surgery, whether due to the need for neoadjuvant therapy or for medical optimization prior to PD, accepting the likelihood that there is likely an increased risk of infectious complications postoperatively (see Table 141-2).
Biliary Stenting in Pancreatic Cancer
| Author | Number of Patients | Morbidity | Mortality | Infectious Complications | Wound Infection | Intra-abdominal Abscess | Fistula |
|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | ||
| Povoski et al21 | |||||||
| Total | 240 | 48 | 5 | 34 | 16 | 14 | 10 |
| Stented | 126a | 55 | 8a | 41a | N/A | 19a | N/A |
| Unstented | 114 | 39 | 3 | 25 | N/A | 8 | N/A |
| Sohn et al22 | |||||||
| Total | 567 | 34 | 1.9 | 29 | 8 | 4 | 8 |
| Stented | 408 | 35 | 1.7 | 32 | 10a | 4 | 10a |
| Unstented | 159 | 30 | 2.5 | 22 | 4 | 6 | 4 |
| Pisters et al23 | |||||||
| Total | 265 | 87 | 1 | 35 | 10 | 8 | 8 |
| Stented | 172 | 88 | 1 | 37 | 13a | 6 | 8 |
| Unstented | 93 | 43 | 2 | 21 | 0 | 5 | 14 |
| van der Gaag et al24 | |||||||
| Total | 202 | 58 | 6.6 | 23 | 10 | 2.5 | 10 |
| Stented | 106 | 75a | 4 | 27a | 13a | 2 | 8 |
| Unstented | 96 | 37 | 9 | 18 | 7 | 3 | 11 |
Regardless of whether neoadjuvant therapy is pursued, indication for surgical resection is contingent upon the ability to achieve an R0 resection. For this, some have proposed the routine use of diagnostic laparoscopy as an adjunct to modern imaging modalities in order to better assess for metastatic disease. The main source of debate relates to which patients should undergo laparoscopy, selective patients with advanced disease or all patients, to detect occult metastatic or advanced pancreatic cancer. In one of the early trials, Warshaw et al25 evaluated the use of laparoscopy in 40 patients diagnosed with PDA. In this series, 43% of patients had metastatic disease at the time of surgery, of which 82% were diagnosed via laparoscopy. While these data were obtained prior to the use of high-resolution imaging, it is argued, therefore, that such numbers would not be duplicated if more modern CT scans were used. In the era of dual-phase CT, Maithel et al26 demonstrated a lower rate of occult metastatic disease on diagnostic laparoscopy in 491 patients felt to have resectable disease. These 491 patients then underwent diagnostic laparoscopy with detailed examination of the liver, peritoneal surfaces, and transverse colon mesentery. Ninety-six patients were unresectable at the time of laparoscopy because of metastasis to the liver (62%), peritoneum (17%), or locoregional spread (22%). Retrospective subset analysis of this population showed that CA 19-9 levels correlated with unresectability. Multivariate analysis revealed a CA 19-9 value greater than 130 U/mL was an independent predictor of tumor unresectability with a hazard ratio of 2.7. While others have studied the use of laparoscopy for staging as a separate procedure independent of laparotomy, much bias exists in these publications. In a 2013 Cochrane Review of 15 studies, the authors concluded that for every 100 patients, 23 would avoid unnecessary laparotomy if diagnostic laparoscopy were routinely performed after CT scan.27 This was based on their collection of data showing that with a negative CT scan, 40% of patients will still be deemed unresectable at the time of laparotomy. Even with a negative laparoscopy, an additional 17% will have metastatic disease at the time of laparotomy. Much of this is due to occult hepatic disease, which becomes evident on manual palpation. Because of this, some have suggested using intraoperative US and a more extensive peripancreatic dissection laparoscopically, but the effectiveness of these modalities is still to be determined. Our practice is to use laparoscopy on a case by case basis, especially in those patients with markedly elevated CA 19-9 levels, large primary tumors (>4 cm), tumors located in the body or tail of the pancreas, or equivocal findings of locally advanced disease on CT imaging, as these patients are high-risk for occult disease at the time of resection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree