Pancreatic Cancer
 ANATOMY AND PATHWAYS OF SPREAD
ANATOMY AND PATHWAYS OF SPREAD
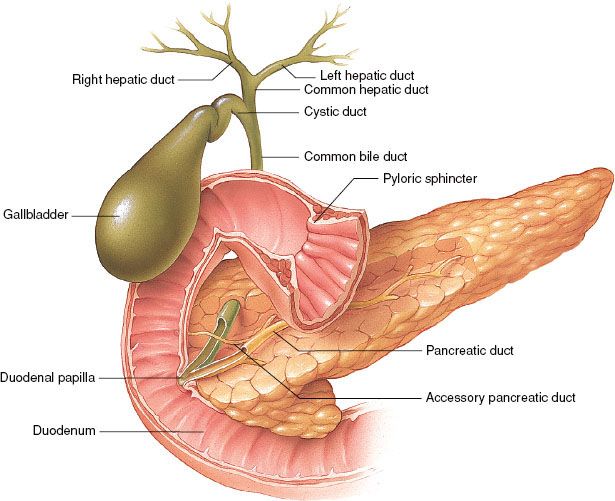
The pancreas lies in the retroperitoneal space of the upper abdomen at about the level of the first two lumbar vertebrae. It is divided into head (including the uncinate process), neck, body, and tail (Fig. 59.1). It has intimate contact with surrounding organs, including stomach, duodenum, jejunum, kidneys, spleen, and major vessels, which can be involved by direct tumor extension. Tumors in the pancreatic head often invade or compress the common bile duct, causing jaundice and dilatation of the bile and pancreatic ducts and gallbladder.
The rich lymphatic drainage of the pancreas is interconnected with duodenal lymphatics. Regional drainage of the pancreatic head is to pancreaticoduodenal, porta hepatis, celiac, and superior mesenteric lymph nodes. The pancreatic body and tail drains to splenic artery, inferior pancreatic, celiac, superior mesenteric, and para-aortic nodal basins.1 With posterior, retroperitoneal tumor extension, para-aortic nodes are at risk. The main venous channels drain via the portal system to the liver. The lungs and pleura may be involved with posterior tumor extension into tissues with venous drainage by the vena cava or its tributaries. Generalized peritoneal involvement is more common with carcinoma of the body and tail than with carcinoma of the head of the pancreas.
 EPIDEMIOLOGY AND RISK FACTORS
EPIDEMIOLOGY AND RISK FACTORS
In 2012 an estimated 43,920 new cases of pancreatic cancer were diagnosed in the United States, with 37,390 estimated deaths from the disease, making pancreatic cancer the fourth leading cause of cancer-related death in the United States.2 At present, surgery offers the only means of cure. Unfortunately, only 10% to 20% of patients present with tumors amenable to resection. Even among patients who present with localized disease, the 5-year overall survival (OS) is approximately 20%, and median survival ranges from approximately 13 to 20 months.3 Contemporary survival rates have reported improved results, particularly in patients with complete surgical resection (R0) and node negative (N0) disease.4–6 Patients who present with locally advanced, unresectable pancreatic cancer have a median survival of approximately 8 to 14 months, with rare long-term survival. Up to 60% of patients present with metastatic disease, which carries a shorter median survival of 4 to 6 months.7
The incidence of pancreatic cancer rises sharply after age 45, with higher rates in males than females (1.3:1) and in black males compared with the general population (14.8/100,000:8.8/100,000).8 Familial aggregation of pancreatic cancer is estimated in 5% to 10% of newly diagnosed cases. It is associated with BRCA1/2 mutations, familial atypical multiple mole melanoma syndrome, Peutz-Jeghers syndrome, familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, hereditary pancreatitis, ataxia telangiectasia, and Li-Fraumeni syndrome, with lifetime risk ranging from 5% to 40%.9,10 Described risk factors for pancreatic cancer include chronic pancreatitis, smoking, alcohol consumption, Helicobacter pylori infection, and factors associated with metabolic syndrome such as obesity and glucose intolerance.11,12
FIGURE 59.1. Pancreas and gallbladder duct anatomy. (Provided by the Anatomical Chart Company.)
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
Most patients with pancreatic cancer experience pain, weight loss, or jaundice. Classically, pain from pancreatic cancer is characterized by radiation to the back, given the organ’s retroperitoneal location. Jaundice, due to biliary obstruction, is often accompanied by pruritus, acholic stools, and dark urine color. The initial presentation varies according to tumor location. Patients with tumors in the pancreatic body or tail usually present with pain and weight loss, while those in the head of the gland typically present with jaundice, steatorrhea, weight loss, and pain. Recent onset of atypical diabetes mellitus, unexplained thrombophlebitis, or history of pancreatitis is often noted. Patients with pancreatic cancer frequently present with unresectable or metastatic disease, in part due to these nonspecific presenting symptoms and the tendency for early invasion and metastases. No effective screening method to detect disease at the localized stage has been identified. Positive physical findings, if any, generally reflect incurable disease. These may include a palpable abdominal mass (i.e., pancreas, liver, or gallbladder due to biliary obstruction), ascites, supraclavicular nodes, or palpable rectal shelf (i.e., peritoneal seeding).
 EVALUATION
EVALUATION
In recent years, significant advances have been achieved in the imaging and staging of pancreatic cancer.13 Currently, the principal diagnostic tools are helical computed tomography (CT) scan, endoscopic ultrasound (EUS), and laparoscopy. Magnetic resonance imaging (MRI) and positron emission tomography (PET) are emerging imaging techniques in initial evaluation of pancreatic malignancies. These tools have facilitated the characterization of the primary tumor (resectable or borderline resectable vs. unresectable) as well as the identification of metastatic disease so patients can be appropriately and reliably triaged to operative and nonoperative therapies.
The most commonly used staging and diagnostic examination tool is abdominal CT scan. Newer generation, multidetector, high-speed helical CT performed with contrast enhancement and thin section imaging allows high resolution, motion-free images of the pancreas and its surrounding structures to be obtained at varying phases of enhancement. This allows for adequate imaging of the pancreas and assessment of metastatic deposits in other intra-abdominal organs such as the liver.14 Over 90% of patients deemed unresectable due to vascular involvement by CT are truly inoperable at time of surgery.15 CT staging is limited, however, in detection of nodal involvement and peritoneal disease. Pathologic confirmation of malignancy is a necessary step prior to initiating therapy for patients with locally advanced tumors; CT can be utilized to facilitate fine-needle aspiration (FNA).
Another tool for staging and diagnosis is EUS. In this procedure, an endoscope with an ultrasound transducer at its tip is passed into the stomach and duodenum, where it provides high-resolution images of the pancreas and surrounding vessels. EUS facilitates FNA without exposing the peritoneum to potential tumor seeding, as may occur with CT-guided biopsy. Sensitivity for EUS is at least comparable to CT, with tumor detection reported as high as 97%.16 An advantage of EUS over CT is the ability to detect small lesions, which might not be well visualized on cross-sectional imaging.17 Interpretation of EUS is highly operator dependent. Frequently, endoscopic ultrasound is performed in conjunction with endoscopic retrograde cholangiopancreatography.18 This combined diagnostic approach allows for staging, therapeutic stenting of the common bile duct when indicated, and diagnostic FNA simultaneously.
A limitation of current imaging techniques is the inability to visualize small (1 to 2 mm) liver and peritoneal implants. Staging laparoscopy has been used preoperatively to assess for intraperitoneal metastases. A recent meta-analysis demonstrated that the adoption of staging laparoscopy and laparoscopic ultrasound will prevent up to 50% of patients from undergoing unnecessary laparotomy.19 Patients with locally advanced disease and involved peritoneal washings or positive peritoneal biopsies have the same prognosis as those with metastatic disease; these patients are more appropriately treated with systemic therapies.20
Advances in MRI, including high-resolution imaging, faster acquisition time, three-dimensional (3D) reconstruction, functional imaging, and MR cholangiopancreatography have led to an improved ability of MRI to diagnose and stage pancreatic cancer.14 This modality can be used in patients with poor renal function to assess the primary tumor and determine resectability; however, studies suggest that MRI is not as sensitive as EUS or CT in tumor detection.21 A potential advantage of MRI is identification of small foci of hepatic metastatic disease difficult to appreciate by CT or to further characterize ill-defined lesions seen on CT.22
Initial studies showed that PET has a higher sensitivity, specificity, and accuracy than CT in diagnosing pancreatic carcinomas.23–24,25 More recent studies have compared integrated PET-CT with PET or CT alone in pancreatic cancers. Lemke et al.23 evaluated 104 patients, with suspected pancreatic lesions. Integrated PET-CT had a higher sensitivity for malignancy detection than either PET or CT alone (96% vs. 84% vs. 77%) but did not improve specificity (64%). PET may also be a useful adjunct in the identification of benign versus malignant lesions and presence of metastatic disease. Although PET-CT may be useful in initial diagnosis, its role in determining resectability has been questioned as coregistration is often performed with lesser resolution CT images and high metabolism can obscure peripancreatic planes.26
High-resolution pancreatic CT and EUS remain the current standard for diagnosis and staging of pancreatic malignancies. Staging laparoscopy is useful in assessing for intraperitoneal and liver disease. MRI and PET-CT are emerging technologies that require further investigation. All image modalities are inadequate to stage lymph node involvement, and further advances may improve the ability to diagnose, stage, and appropriately select further therapies.
 TUMOR STAGING
TUMOR STAGING
Staging is rarely used in published series, and tumors are instead characterized as resectable, unresectable, or metastatic. A new subclassification of localized pancreatic cancer (borderline resectable) has emerged.
The current American Joint Committee on Cancer 2010 tumor, node, and metastasis staging system is described in Table 59.1. Common criteria of resectability are outlined in Table 59.2.
TABLE 59.1 AMERICAN JOINT COMMITTEE ON CANCER STAGING 2010 CLASSIFICATION FOR PANCREATIC CANCER
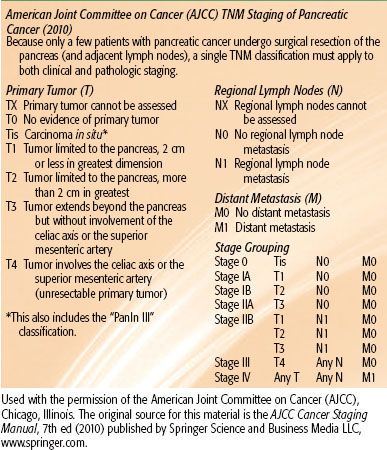
TABLE 59.2 CRITERIA DEFINING RESECTABILITY STATUS

 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
Approximately 85% to 90% of pancreatic cancers are adenocarcinomas. Other neoplasm histological cell types include intraductal papillary mucinous neoplasm, mucinous cystic, solid pseudopapillary, acinar cell, pancreaticoblastoma, neuroendocrine, squamous cell, serous cystadenocarcinoma, and lymphoma. Management and treatment sections will focus on pancreatic adenocarcinomas, given this is the predominate histologic subtype. The histopathology of lesions in the periampullary region of the head of the pancreas is of particular importance as different types of adenocarcinomas, originating from the pancreas, bile duct, ampullary region, or duodenum, respectively, portend different prognoses.27
Pancreatic adenocarcinomas evolve from noninvasive pancreatic intraepithelial neoplasias, acquiring various genetic and epigenetic mutations. Several molecular abnormalities have been implicated in contributing to the development of pancreatic cancer. The most frequent genetic alterations are activation of the oncogene KRAS and inactivation of tumor suppressor genes including TP53, p16 (CDKN2), DPC4 (SMAD4), and BRCA2, with reported incidences ranging from 50% to 95% in pancreatic tumors.28 These molecular changes have the potential to help in identifying precursor lesions with a high likelihood of progressing into carcinomas, characterizing pathologically ambiguous lesions, and useful in development of screening regimens.
 GENERAL MANAGEMENT
GENERAL MANAGEMENT
Operative Considerations
Surgery, as part of a multimodality treatment approach for patients with resectable pancreatic cancer, represents the only potentially curative treatment strategy.29 More than 80% of patients present with advanced disease that is not amenable to curative resection, with roughly two-thirds presenting in the pancreatic head. The standard surgical treatment for pancreatic cancer of the head or uncinate is pancreaticoduodenectomy, first described by Whipple et al.30 in 1935. For pancreatic tail lesions, distal pancreatectomy is employed, frequently with splenectomy. Initially, high operative morbidity and mortality rates led to technical modifications of the operation that, combined with improvements in anesthesia and critical care, have resulted in current perioperative mortality rates of ≤2% at high-volume centers.31–32,33 With reduced morbidity from the Whipple procedure, there is no age limitation, and selected patients, even at ages >80 years, may undergo pancreaticoduodenectomy.34–36
Several studies report low postoperative mortality rates and appropriate patient selection at high-volume institutions, highlighting the importance of performing such procedures at institutions with extensive experience.37–40 In addition, the definition of resectable lesions has broadened with the ability to perform venous reconstructions with minimal increase in morbidity or mortality.41,42 There are no universally accepted criteria for resectability other than absence of distant lymph node involvement or visceral, peritoneal, or extra-abdominal metastases, although the high likelihood of achieving R0 resection should be the ultimate goal.
The prognosis, even among resectable patients, remains poor, with 5-year survival rates of 10% to 25%. The number of nodes harvested appears to impact outcomes; data suggest that 12 to 15 lymph nodes constitutes an adequate assessment, although impact on outcomes may simply reflect accurate staging.43,44 Although appropriate lymph node dissection is desirable, trials of extended lymphadenectomy conveyed no survival benefit and compromised quality of life.45,46 Pathologic assessment provides important prognostic information, including margin status, tumor grade, tumor size, lymphovascular invasion, and lymph node involvement. The presence of positive operative margins, poor histologic grade, larger tumor size, lymphovascular invasion, and lymph node involvement portends a poorer prognosis.47–48,49,50–51 Similarly, patients with elevated pre- and postoperative carbohydrate antigen 19-9 (CA19-9) have inferior disease-free survival (DFS) and OS.51,52,53–54
In an effort to minimize surgical morbidity, pylorus-preserving pancreaticoduodenectomy and laparoscopic resection techniques have developed. Pylorus-sparing pancreaticoduodenectomy potentially improves gastrointestinal function without compromising oncologic management.55,56 Laparoscopic pancreatic resection was first explored in the late 1990s, and its use has increased dramatically.57,58 Although most experiences are small, retrospective, single-institution series, laparoscopic resection, in experienced hands, appears a reasonable option for distal pancreatic resections.59–62 The data on laparoscopic pancreaticoduodenectomy are more limited; the difficulty with this procedure arises from more complex dissection and the need for reconstruction.42,58 However, preliminary data suggest that despite prolonged operative times and potential increase in postoperative fistula formation, laparoscopic resection offers shortened hospitalization and equivalent oncologic results.58,63–65
Cause of Death and Patterns of Failure
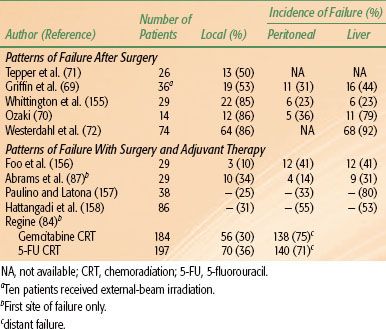
For patients with locally unresectable tumors or metastatic disease, death usually results from hepatic failure secondary to biliary obstruction by local tumor extension or hepatic replacement by metastases. For the 10% to 20% of patients undergoing a potentially curative pancreaticoduodenectomy, three major sites of disease relapse dominate: locoregional, peritoneal cavity, and liver (Table 59.3). High local failure rates of 50% to 86% occur despite resection because of frequent lymphatic and perineural involvement and cancer invasion into the retroperitoneal soft tissues, with an inability to achieve wide retroperitoneal soft tissue margins due to anatomic constraints (SMA and SMV, portal vein, and inferior vena cava).50,66,67 The incidence of microscopic residual disease after careful evaluation of the posterior peripancreatic soft tissue margin is as high as 40%.67 Therefore, tumor stage, grade, and resection margin status are the best predictors of survival after surgery.66,67 The use of adjuvant therapy after surgery can reduce the incidence of local failure, but distant disease recurrence rates remain high.
TABLE 59.3 PATTERNS OF FAILURE AFTER RESECTION OF PANCREATIC CANCER WITHOUT ADJUVANT RADIATION THERAPY OR CHEMOTHERAPY
 RADIATION THERAPY TECHNIQUES
RADIATION THERAPY TECHNIQUES
Dose-Limiting Tissues
The dose-limiting organs for irradiation of upper abdominal malignancies include the small bowel, stomach, liver, kidneys, and spinal cord. Various techniques, such as 3D conformal radiotherapy (3DCRT) and intensity-modulated radiotherapy (IMRT), can spare these organs. Given long-term survival is low, the actual number of patients at risk for late complications is small. Late small bowel and gastric effects may also be decreased through reduction of the volume of these organs within the high-dose field.
Treatment Volumes, Fields, and Doses
In patients undergoing surgery, clips should be placed to mark the extent of the lesion for postoperative irradiation. Used sparingly (e.g., a single small vascular clip placed to mark superior, inferior, lateral, and medial margins), small clips produce only minimal interference on CT scans.
The patient should be positioned supine during simulation and treatment. Until the mid-1990s, conventional simulation was the standard for radiotherapy treatment planning in pancreatic malignancies. During conventional simulation, an initial set of anterior-posterior/posterior-anterior (AP/PA) and cross-table lateral films is obtained after injection of renal contrast medium to identify both operative clips and renal position relative to the field center. Additional films can be obtained with contrast medium in the stomach and duodenal loop in unresected patients.
Three-dimensional conformal radiotherapy represents a major paradigm shift in radiation oncology treatment planning with better visualization of internal organs and target delineation. CT-based treatment planning allows the construction of normal tissue dose–volume histograms and the generation of treatment plans that optimize radiation dose delivery to tumor while sparing critical normal tissues. Despite this advancement, the intent of treatment remains the same. Multiple field, fractionated, external-beam techniques utilizing high-energy photons deliver 45 to 50 Gy in 1.8 Gy fractions to tumor bed, unresected or residual tumor, and lymph node–bearing areas at risk. After 45 to 50.4 Gy, a boost field can be designed to include unresected or gross residual disease, as defined by CT scans and clips, while excluding most of the stomach and small bowel.
With lesions in the head of the pancreas, major node groups include the pancreaticoduodenal, porta hepatis, celiac, and superior mesenteric nodes. Approximately two-thirds of the left kidney must be excluded from the AP/PA field because the right kidney is often in the field due to duodenal (bed) inclusion. The entire duodenal loop with margin is generally included, as pancreatic head lesions may invade the medial wall of the duodenum and place the entire circumference at risk, including the pancreaticoduodenal nodal basins. The superior field extent is often at the middle or upper portion of the T11 vertebral body for adequate margins on the celiac vessels (T12, L1) and the inferior limit at the level L2–3 to include the superior mesenteric lymph nodes and third portion of the duodenum. The upper field extent is sometimes more superior with body lesions to obtain adequate margin on the primary lesion. With lateral fields, the anterior field margin is 1.5 to 2.0 cm beyond gross disease. The posterior margin is often 1.5 cm behind the anterior portion of the vertebral body to allow adequate margins on para-aortic nodes, which are at risk with posterior tumor extension in head or body lesions. The lateral contribution usually is limited to 15 to 18 Gy because a moderate volume of kidney or liver may be in the irradiated volume (Fig. 59.2).
With pancreatic body or tail lesions, at least 50% of the left kidney may need to be included to achieve adequate margins on node groups at risk (i.e., lateral suprapancreatic, splenic artery, and splenic hilum nodes). Inclusion of the entire duodenal loop is often not indicated with body or tail lesions, and at least two-thirds of the right kidney can be preserved. With customized multileaf collimator (MLC) blocking, it is usually possible to cover pancreaticoduodenal and porta hepatis nodes adequately (Fig. 59.3).
After resection, AP/PA and lateral fields are designed on the basis of preoperative CT primary tumor volumes, preoperative duodenal loop location, operative clip placement, and postoperative CT nodal volumes (Radiation Therapy Oncology Group [RTOG] contouring atlas accessible on their website). The anterior border is determined by vascular or nodal boundaries (porta hepatis, superior mesenteric, and celiac), as demonstrated on CT, as well as preoperative tumor location.
FIGURE 59.2. External-beam four-field technique for pancreatic head lesion. A: The anteroposterior/posteroanterior (AP/PA) field, which includes gross tumor (red), duodenal loop (pink) (plus approximately 50% of the right kidney [light green]), liver (brown), and nodal areas at risk (porta hepatis: orange, superior mesenteric arteries: green, celiac: magenta). Most of the left kidney (blue) is excluded from the AP/PA field. B: The right lateral field with an anterior margin beyond gross disease and a posterior margin behind front edge of vertebral body. The liver contour (brown) has been removed from lateral field for visualization of other structures.
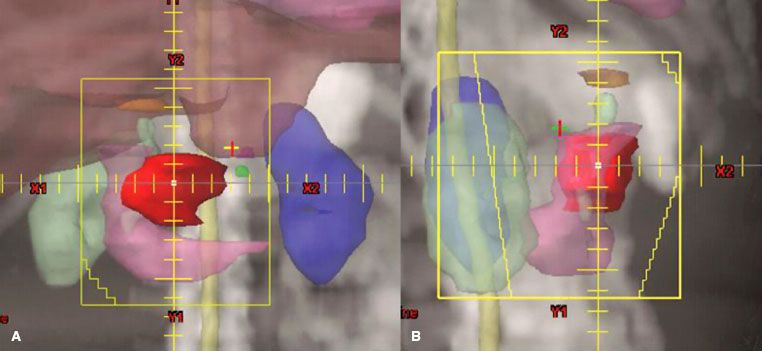
FIGURE 59.3. External-beam four-field technique for unresectable tail of pancreas lesion. A: The anteroposterior/posteroanterior (AP/PA) field showing lesion (red) and head of pancreas (green). The field is extended to the patient’s left to include coverage of the splenic hilum (cyan). Much of the right kidney (light green) is excluded from the field and multileaf collimators block most of the left kidney (blue). B: The right lateral field with an anterior margin beyond gross disease and a posterior margin at least 1.5 cm behind front edge of vertebral body.
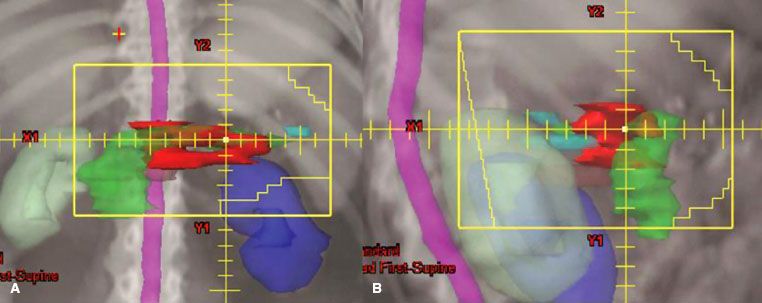
TABLE 59.4 PROSPECTIVE, RANDOMIZED TRIALS FOR ADJUVANT THERAPY FOR PANCREATIC CANCER
 RADIATION THERAPY RESULTS
RADIATION THERAPY RESULTS
Resectable Tumors: Adjuvant Therapy
After surgical resection of pancreatic cancer, local recurrence rates range from 50% to 86% and distant recurrence rates from 40% to 90%, most commonly to the liver or peritoneum.50,67–68,69–72 This provides the rationale for adjuvant therapy, including adjuvant external-beam radiation therapy (EBRT), chemotherapy, and chemoradiotherapy (CRT), which have been employed in an effort to improve patient outcomes (Table 59.4). Despite multiple randomized trials, a definitive role for adjuvant therapy for resected pancreatic cancer has not been established.
Prospective Trials
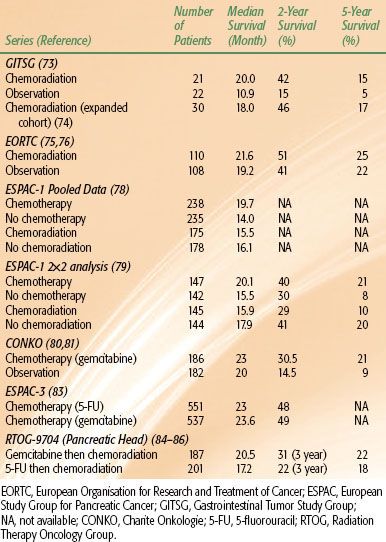
The Gastrointestinal Tumor Study Group (GITSG) conducted the first multicenter prospective trial of adjuvant CRT for patients with resected pancreatic cancer and negative surgical margins, laying the foundation for the adoption of CRT in the United States. Forty-three patients were randomized to observation or CRT to 40 Gy delivered in split-course fashion with concurrent 5-fluorouracil (5-FU) (500 mg/m2) as an intravenous bolus on the first 3 and last 3 days of radiation, followed by maintenance weekly 5-FU for 2 years or until disease progression. At interim analysis, improvements in median DFS and OS with CRT were observed: 11 versus 9 months and 20 versus 11 months, respectively. Two-year OS was 42% in the CRT group versus 15% in the surgery-alone arm.73 An additional 30 patients were later enrolled to receive adjuvant CRT. These additional patients confirmed the survival outcomes seen in the original trial, with median survival of 18 months and a 2-year survival of 46%.74
The GITSG trial has been criticized for a number of reasons. The trial closed prematurely following enrollment of 43 of an intended 100 patients due to slow accrual over the 8-year enrollment period. The EBRT approach in this trial was considered low dose and antiquated by contemporary standards due to a split-course technique and use of large treatment fields, which encompassed the entire pancreas or pancreatic bed and the celiac, pancreaticosplenic, peripancreatic, and retroperitoneal regional lymph nodes. Additionally, the inclusion of both CRT and adjuvant chemotherapy after surgery evaluated two treatment variables, making it difficult to discern the true effect of either treatment alone. In the CRT arm, there were issues of compliance, with 32% of patients assigned to CRT receiving inappropriate radiation and 25% of patients failing to initiate treatment within 10 weeks postsurgery, the protocol-specified time limit. Only 9% of patient completed the planned 2-year maintenance chemotherapy course. In addition, survival in the control arm was low compared to historical controls. Despite these limitations and the failure to reach the desired patient accrual, the GITSG trial demonstrated a benefit for CRT, which became standard adjuvant therapy, particularly in the United States.
A second study sponsored by the European Organisation for Research and Treatment of Cancer (EORTC) sought to confirm the findings of the original GITSG study. Two hundred eighteen patients with resected pancreas (n = 114) or nonpancreas periampullary cancers (n = 104) were randomly assigned to observation or CRT to 40 Gy in a split-dose fashion with concurrent continuous infusional 5-FU (25 mg/kg) without further adjuvant chemotherapy. This study showed no significant improvement (P = .208) in median survival (19 months [observation] vs. 24.5 months [CRT]) or 2-year survival (41% [observation] vs. 51% [CRT]).75 Long-term follow-up of the EORTC trial demonstrated no difference in 5-year OS with CRT use: 22% (surgery alone) versus 25% (CRT). Post hoc analysis of pancreatic head lesions failed to demonstrate a benefit with CRT, with a median OS of 1.3 years for CRT versus 1 year for surgery alone.76
This trial has been criticized for its heterogeneous patient population, which included patients with both pancreatic and other periampullary primary tumors. Periampullary carcinomas have a significantly better prognosis compared with pancreatic cancer; the two entities thus represent truly different diseases and potentially dilutes any evidence of benefit for adjuvant CRT.77 Similar to the GITSG trial, older EBRT techniques—split course and low total dose—were used. Also, >20% of patients in the CRT arm did not receive the intended treatment due to postoperative complications or patient refusal. The trial did not include maintenance chemotherapy in the treatment arm. Finally, although a small subset, patients undergoing noncurative resection and patients with positive surgical margins were still eligible for enrollment. The discordant results of the EORTC and GITSG trials have led some investigators to attribute the OS benefit seen in the GITSG trial to maintenance chemotherapy administration rather than to EBRT.
An additional European trial conducted by the European Study Group for Pancreatic Cancer (ESPAC) aimed to further explore the question of appropriate adjuvant therapy for “macroscopically” resected pancreatic cancers. Treating physicians were allowed to enroll patients into one of three parallel randomized studies:
1. Chemoradiation versus no chemoradiation (n = 68), consisting of 20 Gy over 2 weeks with 5-FU (500 mg/m2) on days 1 through 3, then repeated after a 2-week break;
2. Chemotherapy versus no chemotherapy (n = 188), consisting of bolus 5-FU (425 mg/m2) and leucovorin (20 mg/m2) given for 5 days every 28 days for 6 cycles; or
3. A 2-by-2 factorial design of 285 patients enrolled on chemoradiotherapy (n = 70), chemotherapy (n = 74), chemoradiotherapy with maintenance chemotherapy (n = 72), or observation (n = 69).
The data from the treatment groups from all three parallel trials were then pooled for analysis. There was no survival difference between the 175 patients who received adjuvant chemoradiation and the 178 patients who did not receive therapy (median survival, 15.5 vs. 16.1 months; P = .24). In the chemotherapy arm, however, a 35% reduction in death was seen in the group who received adjuvant chemotherapy (n = 238) compared with those who received no chemotherapy (n = 235), with a difference in median survival of 19.7 versus 14 months (P = .0005).78 On further follow-up of patients randomized using the 2-by-2 factorial design, the 5-year survival rate for the patients who received chemotherapy was 21% versus 8% for those who did not. Additionally, adjuvant CRT was associated with a deleterious effect on survival.79
A number of problems are associated with the interpretation of these data. First, the complex trial design has the potential for bias, because patients or physicians could select randomization for one treatment variable. Also, as in the aforementioned trials, the EBRT technique used was, by contemporary standards, considered outdated, using split course and low total dose. No details of EBRT delivery or central quality assurance for EBRT, surgery, or pathology are available. In addition, many treatment violations occurred, as only 62% of patients received full CRT treatment and only 42% of patients in the chemotherapy arms completed the predefined regimen. Although many have attempted to draw conclusions from the updated publication, the 2-by-2 factorial cohort of the study was not powered to detect OS differences. Additionally, patients receiving CRT in the ESPAC trial experienced poorer survival outcomes compared with those in other reported CRT series. Also, patients who had received prior “background” chemotherapy or radiation therapy were still eligible for enrollment. As in the EORTC trial, no maintenance chemotherapy was administered. Despite these critiques, some have speculated that the detriment seen in the CRT group may have resulted from delayed administration of systemic therapy shown to improve survival in the ESPAC trial.
A common critique of the aforementioned trials of adjuvant therapy is the lack of restaging to evaluate for the presence of persistent or metastatic disease after surgical resection and prior to the initiation of adjuvant therapy. The time between initial staging and the commencement of adjuvant treatment can be as long as 3 to 4 months, during which a significant number of patients would be expected to develop radiographically apparent metastases. Without interval restaging, these patients may inappropriately receive CRT. Although these three trials formed the foundation for adjuvant treatment approaches to resectable pancreatic cancer, perhaps because each trial was fraught with flaws, there continues to be little consensus regarding the “most appropriate” treatment. A relative dichotomy in adjuvant treatment approaches for resectable pancreatic cancer has emerged between the United States and parts of Europe, with adjuvant CRT frequently implemented in the United States and adjuvant chemotherapy alone administered in parts of Europe.
Building from the data showing potential benefit to adjuvant chemotherapy, investigators in Europe conducted a randomized phase III trial of observation versus adjuvant gemcitabine in patients with resected pancreatic cancer. Three hundred sixty-eight patients were enrolled on the Charite Onkologie (CONKO) trial and randomized to observation or adjuvant gemcitabine (1,000 mg/m2) intravenous days 1, 8, and 15 every 4 weeks for 6 months.80 The primary end point was DFS, and patients treated with gemcitabine achieved a statistically significantly longer DFS (14.2 vs. 7.5 months) than those observed after surgery. This improvement was seen in both the R0 and R1 subgroups. At initial publication, there was no difference in OS. With longer follow-up, median and 5-year OS were both improved in patients receiving gemcitabine (23 vs. 20 months, and 21% vs. 9%, respectively).81 These findings were supported by a smaller randomized study from Japan, which again demonstrated a DFS benefit for gemcitabine compared to surgery alone.82 At the same time the CONKO trial was enrolling patients, ESPAC-3, the largest randomized controlled trial in pancreatic cancer to date, enrolled 1,088 patients with pancreatic adenocarcinoma who underwent R0 or R1 resection. Patients were randomly assigned to 6 cycles of adjuvant gemcitabine (3 weekly infusions of 1,000 mg/m2) or 6 cycles of bolus 5-FU (425 mg/m2)/leucovorin (LV) (20 mg/m2). Patients receiving 5-FU/LV experienced significantly higher rates of grade 3 or 4 gastrointestinal toxicity (stomatitis and diarrhea), whereas patients receiving gemcitabine experienced significantly higher rates of grade 3 or 4 hematologic toxicity. At a median follow-up of 34.2 months, there was no difference between the two groups in the primary end point of OS. Given its favorable toxicity profile, in many parts of Europe, gemcitabine alone is considered the standard adjuvant treatment in patients with resectable pancreatic cancer.83 The focus of future European adjuvant therapies for resectable pancreatic cancer has centered on finding the ideal combination of systemic agents. The ongoing ESPAC-4 trial is accruing patients with resected pancreatic cancer for randomization to gemcitabine alone versus gemcitabine/capecitabine; the trial is expected to close in 2014.
In the United States, however, the GITSG trial laid the foundation for CRT as the predominate adjuvant treatment modality. The RTOG/Gastrointestinal Intergroup trial 9704 was a phase III randomized study comparing adjuvant 5-FU–based chemotherapy to gemcitabine-based chemotherapy, with both regimens followed by CRT. Four hundred fifty-one patients with resected pancreatic cancer were randomized to continuous infusion 5-FU (250 mg/m2/day) or gemcitabine (1,000 mg/m2 weekly) for 3 weeks prior to CRT and 12 weeks after CRT. CRT in both groups consisted of 50.4 Gy delivered with continuous infusion 5-FU (250 mg/m2/day).84 Prospective quality assurance of all radiation therapy plans was required. This trial was powered to demonstrate a survival benefit for the entire cohort and for the subgroup of patients with pancreatic head lesions. On initial analysis of the pancreatic head subgroup (n = 388), a nonsignificant trend toward improved median and 3-year OS was seen in the gemcitabine arm: 20.5 versus 16.9 months and 31% versus 22%, respectively. However, a higher incidence of grade 3 or higher hematologic toxicity was also seen in the gemcitabine group, with no significant differences seen in severe nonhematologic toxicities.
An update of this trial reported median and 5-year OS rates in patients with pancreatic head tumors of 20.5 months and 22% in those who received gemcitabine, compared with 17.2 months and 18% in those who received 5-FU. On multivariate analysis, in the subgroup of patients with pancreatic head lesions who received gemcitabine, there was a nonsignificant trend toward improved OS (P = .08).85,86
A secondary aim of RTOG-9704 was to assess the ability of post-resection CA19-9 levels to predict survival. When CA19-9 levels were analyzed in a cohort of 385 patients as a dichotomized variable (<180 IU/mL vs. ≥180 IU/mL, ≤90 IU/mL vs. >90 IU/mL), there was a significant survival difference favoring patients with CA19-9 levels of <180 IU/mL. This corresponded to a 72% reduction in the risk of death.52 Unlike preceding randomized CRT studies, a major strength of the RTOG study was the rigorous, centralized quality control of radiation therapy techniques and delivery. A recent analysis demonstrated that patients treated per study guidelines had a significant survival advantage, indicating the importance of centralized review and treatment technique in this disease.87
The high rates of local failure and need for more efficacious systemic therapy, as well as controversy surrounding the role of CRT is resected patients, led to the design of the current RTOG-0848/EORTC trial. This trial randomly assigns patients with resected pancreatic head adenocarcinoma (stratified based on CA19-9 level, nodal involvement, and margin status) to receive treatment with either gemcitabine alone or gemcitabine combined with erlotinib for 5 cycles. If no progression is seen on restaging following the completion of systemic therapy, patients are further randomized either to receive an additional cycle of the previously administered chemotherapy (for a total of 6 cycles) and no further treatment or to receive CRT (50.4 Gy with concurrent capecitabine or 5-FU) using modern techniques central radiation therapy quality assurance. This trial seeks to answer two primary questions: (a) the role of the small-molecule epidermal growth factor receptor (EGFR) inhibitor, erlotinib, in the adjuvant therapy of pancreatic cancer and (b) the role of CRT in the era of modern chemotherapy, particularly in patients who do not experience early disease progression. This is the only contemporary randomized clinical trial evaluating the role of CRT in the adjuvant setting.
Single Institution Experiences
Reports of single-institution experiences with adjuvant therapy for resected pancreatic cancer have provided additional evidence to the benefit of such. A review of the Mayo Clinic experience reported the outcomes of 472 patients who underwent R0 resection between 1975 and 2005. Despite more adverse prognostic features in patients receiving CRT (higher histologic grade and greater lymph node involvement), median, 2-year and 5-year OS were significantly improved in the CRT cohort compared with patients who received no adjuvant therapy: 25.2 versus 19.2 months, 50% versus 39%, and 28% versus 17%, respectively.88 A larger series from Johns Hopkins compared 908 patients who underwent pancreaticoduodenectomy between 1993 and 2005 and received surgery alone or CRT. Patients receiving CRT experienced a significant improvement in median, 2-year, and 5-year OS: 21.2 versus 14.4 months, 43.9% versus 31.9%, and 20.1% versus 15.4%, respectively.89 A pooled retrospective analysis of nearly 1,100 patients from both institutions demonstrated similar results in favor of adjuvant chemoradiation.90
Data with the highest survival after adjuvant therapy for pancreatic cancer come from a phase II trial conducted at the Virginia Mason Clinic. Forty-three of a planned enrollment of 53 patients were treated with CRT to 50 Gy with 5-FU (200 mg/m2/day) continuous infusion, weekly cisplatin (30 mg/m2), and interferon-α (3 million units) subcutaneously every other day. After completion of chemoradiation, patients received further adjuvant 5-FU (200 mg/m2/day) by continuous infusion.91 The 2-year and 5-year OS rates were 64% and 55%, respectively. Median survival was not reached at time of initial publication. With these encouraging data came significant toxicity, as 70% of patients experienced grade 3+ toxicities and 42% required hospitalization. The American College of Surgeons Oncology Group subsequently opened a multicenter, phase II trial evaluating the Virginia Mason adjuvant regimen. Although survival outcomes were promising (median survival 25 months), the trial closed early after accrual of 89 patients due to high toxicity rates. Ninety-five percent of patients experienced grade 3+ toxicity, and 50% failed to complete the entire treatment protocol.92
Neoadjuvant Therapy
Even after undergoing curative resection for pancreatic cancer, 80% to 85% of patients will recur. In addition, positive margins or nodal disease increases this rate of recurrence to approximately 90%.67,93 The use of neoadjuvant chemoradiation offers an alternative approach to improve on these figures for several reasons:
1. Approximately one-third of patients experience a significant delay or do not receive adjuvant therapy following resection.49,94,95
2. Twenty percent to 40% of patients will be spared the morbidity of resection as their metastatic disease becomes clinically apparent during course of neoadjuvant therapy.96–98
3. Preoperative therapy could theoretically be less toxic and more effective as the chemotherapy and radiation would be given without the postsurgical issues of small bowel in the radiation field, decreased oxygenation and decreased drug delivery to the remaining tumor bed.99,100
4. Patients with local, borderline and unresectable lesions may be able to be downstaged to allow for surgical resection and sterilization of the operative region, which potentially facilitaes R0 resection and reduces the risk of spread during surgical manipulation.
Unlike with adjuvant therapy, no phase III randomized trials of neoadjuvant CRT in resectable pancreatic lesions have been conducted, and the vast majority of data come from phase II and retrospective studies. At the MD Anderson Cancer Center, multiple trials of neoadjuvant 5-FU–based CRT have been performed. The earliest trial treated 28 patients with continuous infusion 5-FU (300 mg/m2/day) and concurrent EBRT to 50.4 Gy over 5.5 weeks. Patients who underwent surgical resection also received intraoperative radiation therapy (IORT). Twenty-five percent of patients had evidence of metastatic disease on preoperative restaging. Fifteen percent had metastatic disease that was found on laparoscopy. For the patients who underwent surgery, median survival was 18 months, and 41% had a pathologic partial response to therapy. However, 33% of patients treated in this study required hospitalization for gastrointestinal toxicity from therapy.101 In an effort to minimize hospitalization, this group subsequently focused on rapid fractionation EBRT. A prospective trial of 35 patients treated with EBRT to 30 Gy (3 Gy per fraction for 10 fractions) with concurrent continuous infusion 5-FU (300 mg/m2/day) found grade 3 nausea and vomiting in only 9% of patients with no grade 4 toxicities. Twenty-seven patients were taken to surgery and 20 patients underwent resection and IORT to 10 to 15 Gy. Locoregional recurrence occurred in only 2 of 20 resected patients. Median survival for patients who underwent surgery was 25 months, with a 3-year survival rate of 23%.102
Trials of neoadjuvant CRT with paclitaxel as a radiosensitizer have been conducted. In radiobiologic models, paclitaxel may result in enhanced radiosensitization through synchronization of tumor cells at G2/M, a relatively radiosensitive phase of the cell cycle, and tumor reoxygenation after apoptotic clearance of paclitaxel-damaged cells. In an MD Anderson Cancer Center study, 35 patients received weekly paclitaxel (60 mg/m2) with concurrent EBRT to 30 Gy. Eighty percent underwent resection with 21% of pathology specimens showing >50% tumor necrosis. The 3-year survival for the patients who underwent preoperative therapy and resection was 28%. Hospitalization was required in 11% of patients for toxicity, primarily nausea and vomiting. These preliminary data show an increased toxicity without a significant improvement in histological response rate or survival.103
The French Fédération Francophone de Cancérologie Digestive/Société Francophone de Radiothérapie Oncologique (SFRO-FFCD) 9704 trial incorporated further radiosensitizing chemotherapy. Forty-one patients received CRT to 50 Gy with 5-FU (300 mg/m2/day) and 2 cycles of CDDP (neoadjuvant cisplatin; 20 mg/m2) followed by surgical resection in patients without progression. Sixty-three percent underwent curative surgery, with 80% obtaining an R0 resection and 50% of specimens showing major histologic response. In patients who underwent curative surgery, median survival was 11.7 months, with a 32% 2-year overall survival.104
The evolution of CRT in the neoadjuvant setting appears to parallel advances made with adjuvant therapy, with transition to gemcitabine-containing regimens. In 2008, two published phase II studies from the MD Anderson Cancer Center evaluated the use of gemcitabine as part of a neoadjuvant regimen. One trial enrolled 86 patients with radiographically resectable adenocarcinoma of the pancreatic head or uncinate. Patients received weekly gemcitabine (400 mg/m2) with 30 Gy EBRT over 2 weeks. After restaging 4 to 6 weeks post-CRT, 73 patients (85%) underwent surgery, with 64 (74%) undergoing successful resection. Median survival in resected versus unresected patients was 34 versus 7 months, with corresponding 5-year OS rates of 36% and 0%, respectively. In patients undergoing pancreaticoduodenectomy, 11% experienced local failure, with distant failure representing the first site of failure in the majority of cases.105
Given the high incidence of distant disease development, a simultaneously conducted phase II trial incorporated induction CDDP and gemcitabine prior to initiation of CRT with gemcitabine. Induction chemotherapy consisted of CDDP (30 mg/m2) and gemcitabine (750 mg/m2) every 2 weeks for 4 cycles followed by 4 weekly infusions of gemcitabine (400 mg/m2) with 30 Gy EBRT. Sixty-two of 90 enrolled patients (78%) underwent surgery and 52 (66%) had successful resection. The median survival for patients undergoing surgery was 31 months, compared with 10.5 months in the unresected patients.106
A recent review of the Surveillance, Epidemiology, and End Results (SEER) database supports the use of neoadjuvant treatment. This analysis included 3,885 patients treated for resectable pancreatic cancer: 70 patients (2%) received neoadjuvant EBRT, 1,478 (38%) received adjuvant EBRT, and 2,337 (60%) were treated with surgery alone. Given that the SEER database does not provide information on administration of chemotherapy, this variable could not be assessed. Median OS was 23 months in patients receiving neoadjuvant EBRT, 17 months with adjuvant EBRT, and 12 months in the surgery-alone cohort.107
Despite the potential advantages and encouraging results using a neoadjuvant CRT approach, no randomized trial results exist comparing neoadjuvant to adjuvant therapy, and its role continues to be investigational. A multicenter, randomized phase II study evaluating neoadjuvant therapy in pancreatic carcinoma has been described. This multinational study is comparing outcomes of patients with resectable disease treated with neoadjuvant gemcitabine-based CRT followed by surgery to outcomes in patients undergoing upfront surgery. Resection is followed by adjuvant gemcitabine-based chemotherapy in both arms.108
Therapy for Locally Advanced Carcinoma
Approximately 30% of patients present with locally advanced carcinoma of the pancreas, comprising a group of patients with an intermediate prognosis between resectable and metastatic disease. These patients have pancreatic tumors that are defined as surgically unresectable but have no evidence of distant metastases. A tumor is usually considered to be unresectable if it has one of the following features:
1. Extensive peripancreatic lymph node involvement and/or distant metastases,
2. Encasement or occlusion of the SMV or SMV/portal vein confluence that is not amenable to reconstruction, or
3. ≥180 degree involvement of the SMA; involvement of the inferior vena cava, aorta, or celiac axis.
Recent advances in surgical technique allow for resection of selected patients with tumors involving the SMV.41,109 Treatment with radiation and chemotherapy increases median survival for patients with locally advanced cancers to approximately 8 to 14 months but rarely results in long-term survival. The therapeutic options of patients with locally advanced pancreatic cancer include chemotherapy alone, CRT, IORT, and more recently EBRT with novel chemotherapeutic and targeted agents. In evaluating the results of these various therapies, it is useful to remember that a median survival of 3 to 6 months has been reported for this subset of patients undergoing palliative gastric or biliary bypass only.110
Prospective Trials
Prospective trials have evaluated the role of EBRT versus CRT, variations in CRT regimens, and CRT versus chemotherapy alone. With conflicting results, there is little consensus as to the appropriate management of locally advanced patients, and all are considered reasonable options (Table 59.5). The Mayo Clinic undertook an early randomized trial in the 1960s in which 64 patients with locally unresectable, nonmetastatic stomach, large bowel, and pancreas adenocarcinoma received 35 to 40 Gy of EBRT alone or with concurrent bolus 5-FU (45 mg/kg). A significant survival advantage was seen for patients receiving EBRT with 5-FU versus EBRT only (10.4 vs. 6.3 months).111
The GITSG followed with a similar study comparing EBRT alone to EBRT with concurrent and maintenance 5-FU. One hundred and ninety-four eligible patients with surgically confirmed unresectable and nonmetastatic pancreatic adenocarcinoma were randomized to receive 60 Gy split course EBRT alone, 40 Gy split course EBRT with 2 to 3 cycles of concurrent bolus 5-FU chemotherapy (500 mg/m2), or 60 Gy split course EBRT using a similar chemotherapy regimen. Patients in the latter groups received 2 years’ maintenance 5-FU (500 mg/m2) after EBRT completion. The EBRT alone arm was closed early as a result of an inferior survival rate. The 1-year survival rate in the two combined modality therapy arms was roughly 40% (with no statistical difference between CRT arms) versus 11% in the EBRT alone arm.112
The Eastern Oncology Group’s trial ECOG-8282 randomized 114 patients to EBRT alone (59.4 Gy) with or without continuous infusion 5-FU (1,000 mg/m2/day) on days 2 to 5 and 28 to 31 and mitomycin-C (10 mg/m2) on day 2. There was no difference in response rates, DFS, or OS with the addition of concurrent chemotherapy. Higher rates of toxicity, primarily hematologic, where noted in the CRT group.113
Further studies sought to determine a more efficacious CRT regimen. A second GITSG trial randomized 157 and analyzed results for 143 eligible patients with unresectable disease to 60-Gy split course EBRT with concurrent and maintenance 5-FU (500 mg/m2) (as in the prior GITSG trial) or 40-Gy continuous course radiation with weekly, concurrent doxorubicin chemotherapy (10 mg/m2), followed by maintenance doxorubicin and 5-FU. A significant increase in treatment-related toxicity was seen in the doxorubicin arm, with no survival difference observed between the two groups (median survival 8.5 vs. 7.6 months). No clinical benefit was seen in substituting doxorubicin for 5-FU.114
TABLE 59.5 PROSPECTIVE RANDOMIZED TRIALS FOR LOCALLY ADVANCED, UNRESECTABLE PANCREATIC CANCER