Pancreatic ductal adenocarcinoma is a genetic disease. This perspective is supported by reproducible patterns of genetic mutations that accumulate during tumorigenesis. These patterns indicate the operation of a selective process favoring the emergence of specific constellations of genetic changes. Individuals who inherit a mutant form of certain genes have an increased risk of developing pancreatic cancer. According to this genetic theory, most pancreatic cancers share a common foundation of genetic mutations disrupting specific cellular regulatory controls. These shared abnormalities are responsible for the processes of growth, invasion, and metastasis in individual patients.
Four categories of mutated genes play a role in the pancreatic tumorigenesis: oncogenes, tumor-suppressor genes, genome-maintenance genes, and tissue-maintenance genes (summarized in
Table 17.1). Some of these mutations are germ line, for example, they are transmitted within a family. Genetic mutations acquired during life, termed
somatic mutations, contribute to tumorigenesis within a tissue but are not passed to off-spring.
Very recently, techniques were developed to sequence all of the genes of individual cancers. Whole-exomic sequencing of pancreatic ductal cancers revealed an average of 63 somatic mutations per tumor.
1 Most of these mutations undoubtedly were nonfunctional “passenger” mutations, each mutated at a low frequency and not contributing to tumorigenesis. Indeed, most passenger mutations might arise as a normal aspect of tissue aging before tumorigenesis begins.
2 Smoking is associated with a doubling of the risk for pancreatic cancer, and remarkably is also associated with a 40% increase of the prevalence of low-frequency mutations in the cancers.
3 A subset of the mutations, however, is responsible for “driving” the neoplastic process in the ducts and is the focus here.
Telomere abnormalities and signs of chromosome instability are the most common alterations in pancreatic neoplasia. Four genes are mutated in most pancreatic cancers: the KRAS, p16/CDKN2A, TP53, and SMAD4 genes. Other genetic abnormalities are seen at a much lower frequency, including mutations in the genes BRCA2, PALB2, FANCC, FANCG, FBXW7, BAX, RB1, the TGFβ (transforming growth factorbeta) receptors TGFBR1 and TGFBR2, the activin receptors ACVR1B and ACVR2, MKK4, STK11, GUCY2F, NTRK3, EGFR, and cationic trypsinogen, alterations in the mitochondrial genome, amplifications, various chromosomal deletions, inactivation of DNA mismatch-repair genes, and rarely the presence of the Epstein-Barr virus genome as an episome.
Knowing the genes mutated in a cancer can have direct clinical impact. For example, many cancers occur from an inherited mutation, and these patients and their families could benefit from genetic counseling.
4,
5,
6,
7 A distinct morphologic subtype of pancreatic cancer, the medullary cancer, can suggest such an inherited mutation.
8,
9 Another example includes the analysis of the genetic alterations in precursors to invasive pancreatic neoplasia, which has indicated that most carcinomas arise by a process of progressive intraductal tumorigenesis.
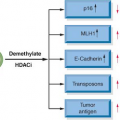
10 Epigenetic changes in DNA methylation and in gene expression are also highly specific for the cancerous cells and can serve as diagnostic markers.
COMMON MOLECULAR CHANGES
Telomere shortening is the earliest and most prevalent genetic change identified in the precursor lesions.
11 Telomere shortening is thought to predispose to chromosome fusion (translocations) and the mis-segregation of genetic material during mitosis.
12 Later during tumorigenesis, telomerase is reactivated,
13,
14 moderating the telomere erosive process while permitting continued chromosomal instability.
15The
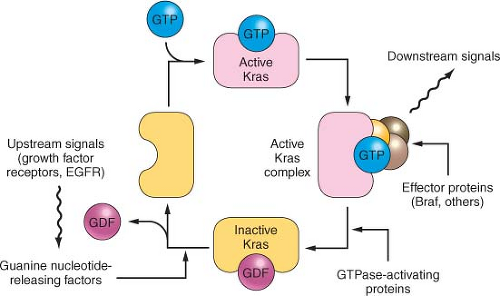
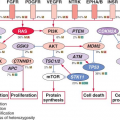
KRAS gene mediates signals from growth factor receptors and other signaling inputs (
Fig. 17.1). The mutations convert the normal Kras protein (a protooncogene) to an oncogene, causing the protein to become overactive in transmitting the growth factor-initiated signals.
16 KRAS is mutated in over 90% of conventional pancreatic
ductal carcinomas.
17 The first genetic change in the ducts is probably not (or not always) a
KRAS gene mutation, for the prevalence of this mutation is highest in the more advanced lesions (
Table 17.1).
18,
19 KRAS is one of a family of
RAS genes that can harbor mutations in human cancers. The other
RAS genes include
NRAS and
HRAS, although it is possible that only
KRAS is mutated in pancreatic carcinomas.
As one of the most commonly mutated genes in pancreatic cancer, Ras is an attractive target for the development of gene-specific therapies, and an understanding of the normal biology of the Ras protein should help in the development of these Ras targeted therapies. The Ras proteins require an attachment to the plasma membrane for activity. For many proteins, including Ras, a hydrophobic prenyl group is essential for the attachment. Either farnesyl (15-carbon) or geranylgeranyl (20-carbon) makes a covalent thioether linkage at a cysteine residue located near the C-terminal end of Ras proteins, termed the CAAX motif. Working mostly in artificial legacy models of the HRAS oncogene (rather than the more widely available but experimentally less tractable natural KRAS-mutant cancer cell lines), the farnesylation reaction was readily inhibited by various means; in these models, the Ras protein was rendered inactive and often accompanied by cytotoxicity limited to the mutant cells.
Although many types of compounds capable of blocking the farnesyltransferase enzyme were
developed as drugs, they have not been successful anticancer agents. There are many reasons for this. Although Hras protein is linked predominantly through farnesyl groups, the Kras protein can be alternately prenylated by geranylgeranyl linkages. Unfortunately, the latter type of linkage is thought to be critical for a wider number of cellular proteins, and for fear of excessive toxicity, geranylgeranyl linkages have not usually been considered as an attractive drug target. Kras protein also appears to bind more tightly than Hras to the farnesyltransferase enzyme, requiring higher drug concentrations.
16 Additionally, the artificial models usually employed the engineered overexpression of the Ras protein, a situation in which the unattached Ras proteins would serve as a dominant-negative inhibitor, binding the necessary interacting proteins and sequestering them in the cytoplasm to ensure the inactivation of all three Ras pathways. Such a concentrationdriven mechanism would presumably not occur under the normal levels of Ras proteins present in human cancers.
20 Indeed, it is proposed that the limited efficacy of farnesyltransferase inhibitors observed in some experimental models and in clinical trials may be attributable to a cellular target not yet identified.
21 Attention has turned to compounds that target the downstream mediators, such as Raf and Mek protein kinase inhibitors.
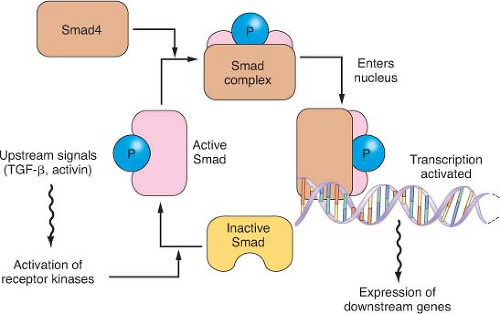
The Smad pathway mediates signals initiated on the binding of the extracellular proteins TGF
β and activin to their receptors (
Fig. 17.2). These signals are transmitted to the nucleus by the Smad family of related genes, including
SMAD4 (DPC4).
22 Smad protein complexes bind specific recognition sites on DNA and cause the transcription of certain genes.
23 Mutations in the
SMAD4 gene are found in nearly half of pancreatic carcinomas, including both homozygous deletions and intragenic mutations combined with loss of heterozygosity (LOH).
24 Other Smad genes are also mutated occasionally.
1Homozygous deletions and mutation/LOH affecting the
TGFβ receptor genes are seen in a few pancreatic cancers.
25 A more common abnormality, in pancreatic as well as in other tumor types, is the underexpression of TGF
β receptors, which results in cellular resistance to the usual suppressive effects of the TGF
β ligand.
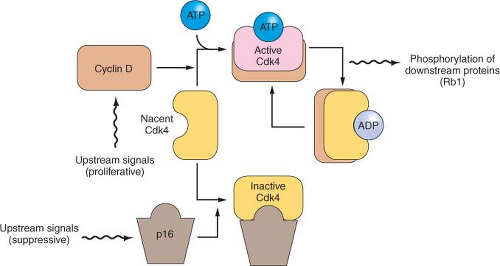
26The
p16/RB1 pathway is a key control of the cell division cycle (
Fig. 17.3). The retinoblastoma protein (Rb1) is a transcriptional regulator and regulates the entry of cells into S phase. A complex of cyclin D and a cyclin-dependent kinase (Cdk4 and Cdk6) phosphorylates and thereby regulates Rb1. The p16 protein is a Cdk-inhibitor that binds Cdk4 and Cdk6.
27,
28,
29 Virtually all pancreatic carcinomas suffer a loss of
p16 function, through homozygous deletions, mutation/LOH, or promoter methylation of the
p16/CDKN2A gene associated with a lack of gene expression.
30,
31 In addition, inherited mutations of the
p16/CDKN2A gene cause a familial melanoma/pancreatic cancer syndrome known as familial atypical multiple mole melanoma.
32,
33,
34,
35,
36 Occasional pancreatic cancers have inactivating mutations of the
RB1 gene.
37