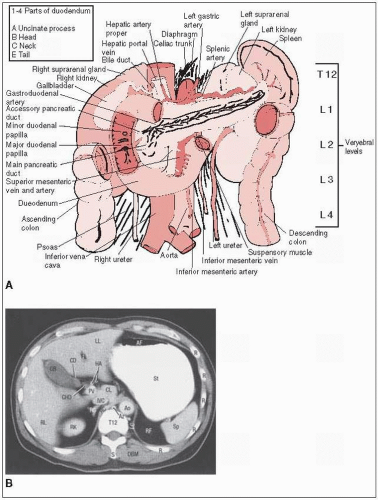
Tumors in the head of the pancreas commonly invade or compress the common bile duct, causing jaundice and dilatation of the bile ducts and gallbladder.
Primary lymphatic drainage is to superior and inferior pancreaticoduodenal, porta hepatis, celiac, and superior mesenteric nodes.
Jaundice, pain, anorexia, and weight loss are the most common presenting symptoms.
Generalized peritoneal involvement is more common with carcinoma of the body and tail than with carcinoma of the head.
The incidence of liver or peritoneal metastases is higher for lesions in the body and tail (75%) than in the head (33%).
For diagnostic purposes and staging, a computed tomography (CT) scan and an endoscopic ultrasound (EUS) are used to best characterize the primary tumor, while preoperative staging laparoscopy can be used to evaluate for intraperitoneal metastases. Magnetic resonance imaging (MRI) and MR cholangiopancreatography (MRCP) have also been used more recently to diagnose and stage pancreatic cancer.
The biliary tract component can be evaluated by transhepatic cholangiography and endoscopic retrograde cholangiopancreatography (ERCP). ERCP typically demonstrates obstruction of the common bile duct as well as a long, irregular stricture in the pancreatic duct. Obstruction of the bile duct and pancreatic duct results in dilatation of both; this is commonly referred to as the “double duct” sign. Cytologic assessment of pancreatic duct brushings may provide a definitive diagnosis, with sensitivity rates of 33% to 62% and specificity rates approaching 100%.
EUS has an accuracy of 75% to 92% in identifying pancreatic neoplasms and can guide the use of fine-needle aspiration biopsy. Magnetic resonance cholangiopancreatography is a noninvasive alternative to ERCP. In a study by Magnuson et al. (17) involving 25 patients with peripancreatic cancer, magnetic resonance cholangiopancreatography identified the level of biliary obstruction in 96% and correctly predicted malignancy in 84%.
The tumor marker CA 19-9 is also helpful in the diagnosis and follow-up of patients with pancreatic cancer. CA 19-9 levels above the upper normal limit of 37 U per mL have 80% accuracy in identifying patients with pancreatic cancer. The accuracy improves to up to 95% when the cutoff value is increased to 200 U per mL (15).
TABLE 27-1 American Joint Committee TNM Staging System for Pancreatic Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Standard surgical treatment for pancreatic cancer is the pancreatoduodenectomy, first described by Whipple et al. (29) in 1935. Criteria for surgical resectability include no distant metastases, a clear fat plane seen around celiac and superior mesenteric arteries (SMA), and a patent superior mesenteric vein (SMV)/portal vein. Factors that would make patients inoperable include extensive peripancreatic lymph node involvement and/or distant metastases, encasement or occlusion of the SMV or SMV/portal vein confluence, or direct involvement of the SMA, inferior vena cava (IVC), aorta, or celiac axis.
For patients with unresectable tumors or metastatic disease, death usually results from hepatic failure due to biliary obstruction by local tumor extension or hepatic replacement by metastases.
For the small number of patients (10% to 20%) undergoing a potentially curative pancreatoduodenectomy, the three major sites of disease relapse are the bed of the resected pancreas (local recurrence), the peritoneal cavity, and the liver.
High local failure rates of 50% to 86% occur despite resection. This is due to frequent cancer invasion into the retroperitoneal soft tissue, as well as the inability to achieve wide retroperitoneal soft tissue margins because of anatomic constraints to wide posterior excision (superior mesenteric artery and vein, portal vein, and inferior vena cava) (30).
For resectable tumors, the Gastrointestinal Tumor Study Group (4) showed that adjuvant 40-Gy split course over 6 weeks with 5-fluorouracil (5-FU) could prolong median survival from 10.9 months to 21.0 months (2-year survival, 18% to 46%). For unresectable pancreatic cancer, the Gastrointestinal Tumor Study Group (23) also showed that a similar chemoirradiation regimen yielded median survival of 9.6 months versus 5.2 months with 60 Gy in 10 weeks alone.
The European Organization for Research and Treatment of Cancer (EORTC) performed a subsequent study, randomizing 218 patients with resected adenocarcinoma of the pancreas or periampullary cancers to 40 Gy of external beam radiation therapy (EBRT) in a split-dose fashion with continuous infusional 5-FU or observational alone. No significant improvement was seen in median survival (24 months versus 19 months p = 0.208). Median survival for pancreatic adenocarcinoma was 17.1 versus 12.6 months (p = 0.09) (11).
The European Study Group for Pancreatic Cancer trial allowed physicians to enroll patients with resected adenocarcinoma of the pancreas onto one of three parallel randomized studies. (a) Chemoradiation versus no chemoradiation (n = 69), with 40-Gy EBRT in split-dose fashion and bolus 5-FU. (b) Chemotherapy versus no chemotherapy (n = 192), with the treatment arm receiving bolus 5-FU and leucovorin, or (c) a 2 by 2 factorial design (n = 289) of chemoradiation, chemotherapy, chemoradiation with maintenance chemotherapy, or observation alone. After pooling all three trials, analysis showed no survival difference between adjuvant chemoradiation versus no therapy (median survival, 15.5 months and 16.1 months, p = 0.24). A survival benefit was seen, however, in those patients who received chemotherapy compared to those who did not (median survival, 19.7 months versus 14 months, p = 0.0005) (19).
In the Radiation Therapy Oncology Group and GI Intergroup Trial 9704, 538 patients received protracted infusion 5-FU and postoperative radiation (50.4 Gy) in 28 fractions and were then randomized to multiple cycles of either infusion 5-FU or gemcitabine hydrochloride (24). There was a survival advantage seen in the patients with resected pancreatic head carcinomas who received maintenance gemcitabine. Median survival was 20.6 months versus 16.9 months in the 5-FU/chemoRT alone arm.
For locally advanced (surgically unresectable) pancreatic cancers, conventional EBRT + 5-FU has been shown to improve survival modestly compared to EBRT or chemotherapy alone.
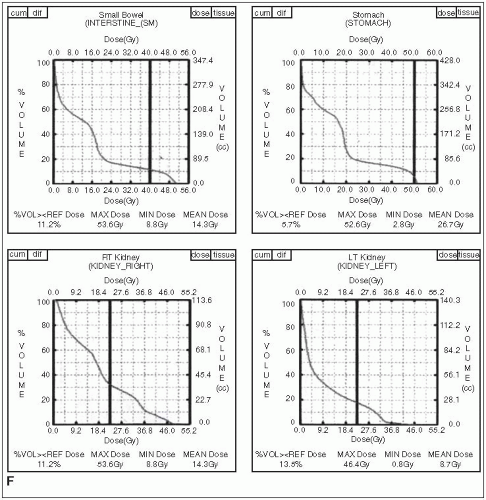
The dose-limiting organs for irradiation of upper abdominal cancers are the small intestine, stomach, liver, kidneys, and spinal cord.
In patients undergoing surgery, clips should be placed to mark the extent of the lesion for later external irradiation.
The patient should be supine during simulation and treatment.
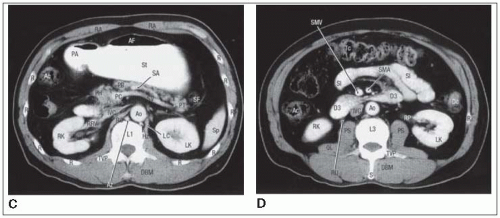
An initial set of anteroposterior (AP) and crosstable lateral films is obtained after injection of renal contrast to identify operative clips and renal position relative to the field center. Additional films may be obtained with contrast in the stomach and duodenal loop.
The intent of treatment is to use multiple-field, fractionated external-beam techniques with high-energy photons to deliver 45 to 50 Gy in 1.8-Gy fractions to unresected or residual tumor, as defined by CT and clips, and to nodal areas at risk.
With lesions in the head of the pancreas, major node groups include the pancreaticoduodenal, porta hepatis, celiac, and suprapancreatic nodes.
The suprapancreatic node group is included with the body of the pancreas for a 3- to 5-cm margin beyond gross disease, but more than two thirds of the left kidney is excluded from the AP-posteroanterior (PA) field because at least 50% of the right kidney is often in the field because of duodenal inclusion.
The entire duodenal loop with margin is included because pancreatic head lesions may invade the medial wall of the duodenum and place the entire circumference at risk (Fig. 27-1).
With pancreatic body or tail lesions, at least 50% of the left kidney may need to be included to achieve adequate margins and include node groups at risk (lateral suprapancreatic and splenic hilum). Because inclusion of the entire duodenal loop is not indicated with these
lesions, at least two thirds of the right kidney can be preserved, but with tailored blocks, it is usually possible to cover pancreaticoduodenal and porta hepatis nodes adequately (Fig. 27-2).
For head of pancreatic lesions, the superior field extent is at the middle or the upper portion of the T11 vertebral body for adequate margins on the celiac vessels (T12, L1).
The upper-field extent is occasionally more superior with body lesions to obtain an adequate margin on the primary lesion.
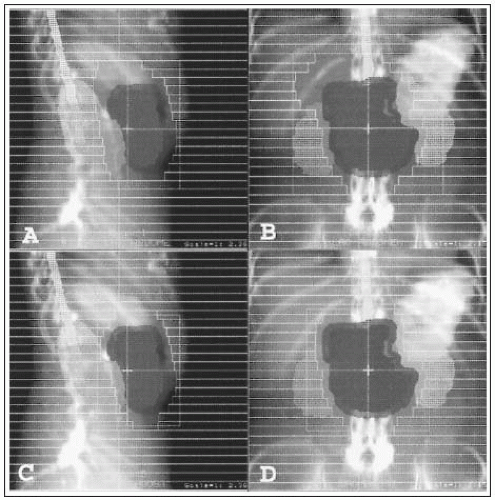
FIGURE 27-2 Three-dimensional planning for four-field external-beam irradiation for the tumor shown in Figure 27-1. A and B: Initial portal to cover the gross tumor and margins for set-up error for 45 Gy in 25 fractions. The field is extended to the right to cover the pancreaticoduodenal and porta hepatis nodes and at least two thirds of the right and left kidneys are shielded. C and D: Boost fields for additional 9 Gy in 5 fractions.
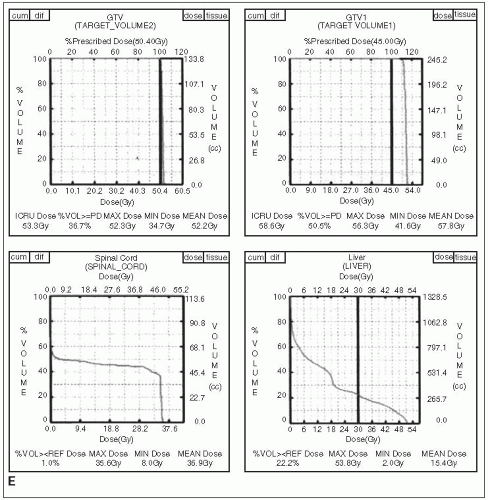
FIGURE 27-1 (Continued) E and F: Dose-volume histograms show target coverage and normal tissue sparing. (Courtesy of Dr. Wade Thorstad.)
With lateral fields, the anterior-field margin is 1.5 to 2.0 cm beyond gross disease. The posterior margin is at least 1.5 cm behind the anterior portion of the vertebral body to allow adequate margins on para-aortic nodes, which are at risk with posterior tumor extension in head or body lesions.
The lateral contribution is usually limited to 18 to 20 Gy because a moderate volume of kidney or liver may be in the field (Fig. 27-2).
After resection, AP-PA and lateral fields are designed on the basis of pre-resection CT primary tumor volumes, operative clip placement, and postoperative CT nodal volumes (14).
The only border that can be more restrictive is the anterior border on lateral fields, since the primary tumor has been resected. This border is determined by vascular or nodal boundaries as demonstrated on CT (porta hepatis, superior mesenteric, and celiac).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree