Summary of Key Points
- •
Palliative radiotherapy is an effective and well tolerated treatment for palliation of thoracic and other symptoms in patients with lung cancer (both nonsmall cell lung cancer and small cell lung cancer).
- •
There is ample high-quality evidence from many randomized trials that short courses (one or two fractions only) of thoracic radiation provide high rates of symptomatic relief.
- •
There is ongoing controversy about whether moderate dose radiation schedules are superior to shorter courses in terms of symptom control or survival benefit; they do result in more toxicity.
- •
Single-fraction palliative radiation is recommended for uncomplicated bone metastases, with high rates of pain relief.
- •
Dexamethasone at a low dose administered on the day of single-fraction radiation for bone metastases and for 4 subsequent days significantly reduces the incidence of pain flare with tolerable side effects.
- •
Stereotactic radiotherapy is emerging as an option for management of patients with vertebral body metastases; trials are in progress to compare it with conventional palliative radiation.
- •
Brain metastases are common in patients with lung cancer; options for management include whole-brain radiotherapy, stereotactic radiation, surgical resection, observation, or best supportive care. Prediction tools can be helpful in identifying patients who have better versus poorer prognosis, in order to guide most appropriate therapy.
Despite substantial advances in earlier diagnosis, with prompt staging and management, and with the improved results of lung cancer treatment, metastatic disease is either present at the time of diagnosis or will develop after initial curative treatment in many patients with lung cancer. A minority of patients with metastases outside the thorax may be candidates for more aggressive multimodality treatment, such as patients with a solitary brain metastasis. However, virtually all patients with stage IV disease and indeed the majority with stage III disease will not be cured, despite the increase in therapeutic options including targeted therapy and systemic chemotherapy that may indeed extend survival and improve or control their cancer for a period of time.
For most patients with metastases, success would be defined as prolonged survival with good quality of life; good performance status; few symptoms of disease; and then, when all options have been exhausted, a peaceful and comfortable death. As a result, the aims in treating patients with incurable disease are prolonging survival, maintaining or improving quality of life, and controlling any cancer-related symptoms with minimum toxicity and inconvenience. Palliative radiotherapy was one of the first treatments to provide symptom improvement for patients with either locally advanced thoracic or metastatic disease, and it continues to have a favorable therapeutic index. The side effects are often minor, and the benefit in terms of symptom improvement is substantial. This finding has been well documented for patients with symptomatic intrathoracic disease and for patients with bone metastases. The evidence for the effectiveness of palliative radiotherapy for patients with lung cancer with brain metastases has been debated in the literature, but it is still the mainstay of treatment for these patients. Palliative radiotherapy is also widely used for metastases at other sites, such as lymph nodes, skin or subcutaneous nodules, adrenal metastases, liver metastases, and orbital or retinal metastases, although there is little formal evidence, other than clinical experience, for its use. Local symptoms may be improved regardless of the histologic subtype of tumor. Small cell lung cancer (SCLC) responds quite rapidly to radiotherapy, and symptoms will improve in most patients. Even though the biology of SCLC, with its tendency to grow rapidly and metastasize, means that chemotherapy is usually the treatment of choice, palliative radiotherapy undoubtedly has a place in treatment management and should not be overlooked. In patients with nonsmall cell lung cancer (NSCLC), the response to palliative radiation is more variable, although symptom response does not directly correlate with radiographic responses and can be seen quite early; e.g., hemoptysis can resolve 12 hours to 24 hours after large-dose-per-fraction radiotherapy, and similarly bone pain may respond in that time frame as well, particularly after single-fraction radiotherapy. Whether response is related to radiation effect on cancer cells, or tumor vasculature, or the surrounding cell matrix with apocrine and metacrine effects or other factors, is far from understood. Elucidating the precise mechanism by which palliative radiotherapy leads to symptom palliation would be a major advance in management of patients with incurable cancers.
This chapter covers the indications for palliative radiotherapy, dose fractionation, planning issues, outcomes including symptoms, quality-of-life benefits and potential effects on prolonging survival, toxicity, and potential repeat treatment issues in patients with lung cancer with either thoracic (lung or mediastinal lymph nodes) or metastatic disease, specifically bone, brain, and other symptomatic sites. Most of the evidence relates to NSCLC, but as mentioned, SCLC would be expected to have a greater symptomatic response to palliative radiotherapy, although a less certain overall benefit in terms of length of disease control.
Palliative Radiotherapy for Thoracic Symptoms
The aim of palliative radiotherapy is to relieve symptoms as completely and as durably as possible. But, as with any palliative treatment, there is a trade-off between the burden of the treatment itself—inconvenience and acute and potentially long-term side effects—and the benefits in terms of symptom relief, quality of life, and, perhaps, improved survival. Evidence-based clinical practice guidelines published in 2012 detail the evidence and recommendations for the role of radiotherapy in thoracic symptom palliation; there is also evidence that international patterns of practice differ.
Indications
Palliative thoracic radiotherapy is indicated whenever a patient has disease that is not potentially curable and has troublesome symptoms that can be attributed to growth of the primary tumor and/or mediastinal node disease. These findings need to be integrated into the whole care of the patient and any systemic therapy that is ongoing or being planned. Thoracic palliative radiotherapy is most often used when a patient is unfit for chemotherapy because of poor performance status or comorbidities and there is no appropriate targeted therapy, or when symptoms persist or recur during or after systemic therapy. Additionally, for patients with NSCLC, because the response to radiotherapy is faster and more reliable than to chemotherapy, there are often occasions when local symptoms mean that it is better to treat patients with radiotherapy first and consider systemic treatment later. For patients with SCLC, palliative radiotherapy is probably only indicated as a first-line treatment in emergency situations such as severe stridor or if the patient is considered too unwell to tolerate systemic chemotherapy, but is often useful when there is symptomatic recurrence and resistance to chemotherapy.
Most of the common symptoms from intrathoracic disease are well palliated by radiotherapy. These include cough, hemoptysis, and chest pain. The response rates range from 50% to 90%, with the highest complete response rates seen for hemoptysis. Breathlessness appears to be less well palliated as it may be also caused by a number of other factors, such as obstructive airways disease, cardiac disease, lymphangitis carcinomatosa, or pleural effusion. Moreover, when there is a tumor in an airway causing lung, lobar, or sublobar atelectasis, the lung may not always reexpand even if the tumor shrinks and the airway is opened up, especially if there has been infection or a coexisting pleural effusion. However, any patient who presents with stridor or computed tomography (CT) evidence of a tumor threatening a major airway should be considered for urgent radiotherapy, as even modest shrinkage of a tumor causing obstruction of a large airway can provide considerable symptom relief. Superior vena cava obstruction (when due to tumor compression rather than thrombus) may also be relieved by palliative radiotherapy, although when severe, more rapid improvement may follow a stenting procedure. However, if a patient has both superior vena cava obstruction and airway compromise, the placement of a superior vena cava stent would not palliate the airway compromise, whereas palliative radiotherapy could improve both; thus, the pros and cons regarding placement of a stent followed by radiotherapy rather than palliative radiotherapy alone need to be carefully considered.
Sometimes it may be considered appropriate to treat patients prophylactically before symptoms develop. There no good evidence that this approach improves outcomes. The authors of one randomized clinical trial carried out in the United Kingdom before widespread use of chemotherapy for patients with NSCLC found that in a group of asymptomatic patients randomly assigned to no immediate palliative radiotherapy, 56% died without having had radiotherapy and that there was no substantial difference in symptom control or survival between patients who received immediate or as required palliative radiotherapy, neither at an early time point nor a later 6-month time point.
Palliative radiotherapy may be delivered either by external beam or as intracavitary; i.e., endobronchial brachytherapy.
External-Beam Radiotherapy
Most palliative radiotherapy to the chest can be delivered simply with uncomplicated fields and short courses of mega-voltage radiotherapy. Because this treatment is palliative and the aim is to improve the patient’s symptoms and improve quality of life, unnecessarily complex and prolonged courses of treatment should be avoided, especially in patients with a poor performance status and limited life expectancy.
Radiotherapy Planning
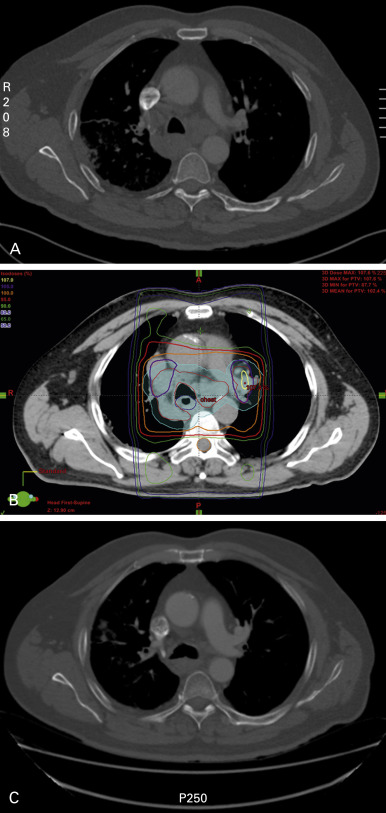
Usually simple parallel opposed anterior/posterior beam arrangements are sufficient, and although recent CT images are useful to assess the extent of disease, complex CT planning should not be needed. The fields should be designed to cover the extent of the tumor causing the troublesome symptoms, but should be kept as small as possible to minimize toxicity ( Fig. 41.1 ), especially as many patients with advanced lung cancer have coexisting chronic obstructive pulmonary disease and therefore already compromised pulmonary function. Widespread sites of disease that are unlikely to be causing symptoms, such as involved but relatively small nodes in the mediastinum, should not necessarily be included. More complex field arrangements may occasionally be needed if there is a risk of overlap with previously treated areas or it is considered essential to minimize the dose to the spinal cord (see later).
Radiotherapy Regimens
The regimens for palliative radiotherapy evolved pragmatically over the past 20 years before they were subjected to any rigorous evaluation in randomized controlled trials ( Table 41.1 ). A number of randomized controlled trials have compared various regimens for palliation and these have been summarized and reviewed in two systematic reviews. These reviews included 14 randomized controlled trials meeting the selection criteria (more than 3000 patients) but use different approaches to incorporating different dose/fractionation regimens in a meta-analysis. For patients with a poor performance status (2–4) there is no evidence that longer, more fractionated regimens provide better palliation or longer survival than shorter regimens, such as 10 Gy in a single fraction or 16 or 17 Gy in two fractions over 1 week. For patients with a good performance status (0 or 1) the evidence is more uncertain, but it is possible that higher dose, more fractionated regimens (such as 36 to 39 Gy in 12 or 13 fractions) provide more durable symptom control and longer survival than lower dose regimens, but at the expense of more toxicity, especially esophagitis (see later). However, the increase in 1-year survival is likely to be modest—assuming a 1-year survival of 45% in this group of patients with better prognosis, the increase is likely to be around 10%, similar to that achieved with cisplatin-based chemotherapy.
| Dose (Gy) | No. of Fractions | No. of Days | BED (Gy10) |
|---|---|---|---|
| 10 | 1 | 1 | 20 |
| 16 | 2 | 8 | 29 |
| 17 | 2 | 8 | 31 |
| 20 | 5 | 5–7 | 28 |
| 30 | 10 | 12–14 | 39 |
| 36 | 12 | 14–16 | 47 |
| 39 | 13 | 15–17 | 51 |
However, although shorter courses of palliative radiotherapy are almost certainly as effective as more prolonged ones for most patients, especially patients who are less well, there appears to be a reluctance to use such regimens in many parts of the world. This reluctance may be because of unfamiliarity, lack of exposure during training, concerns about the risks associated with using large fractions (see later) and the ability to retreat after larger doses per fraction, or unrealistic expectations about the anticipated life expectancy of the patient and possible survival benefits from longer courses. But this reluctance is also influenced by departmental policy and a widespread belief that what is commonly prescribed is indeed the best, and sometimes is also influenced by financial considerations. It is therefore important to consider the burden placed on patients and their families or caregivers from a prolonged course of treatment with repeated hospital visits, when useful palliation can be provided by only one or two fractions.
Side Effects
Typically, palliative thoracic radiotherapy is well tolerated with few toxicities, none of which is likely to be life-threatening. This finding is in marked contrast with the potentially severe and persistent toxicity caused by chemotherapy. The most common side effects of palliative thoracic radiotherapy are fatigue and esophagitis. The severity of esophagitis is generally dose-related, and in most patients it is easily managed and resolves within a week or so. Radiation pneumonitis may occur if large fields and higher doses are used, and care should be taken to minimize the dose to normal lung in those circumstances. In cases of symptomatic radiotherapy pneumonitis, corticosteroids may improve the symptoms of cough and dyspnea, but need to be tapered gradually in order to prevent relapse.
Larger fraction palliative radiotherapy may be associated with specific side effects that have been described by a number of authors. In the first 24 to 48 hours after treatment, up to 50% of patients may experience nausea, brief episodes of acute chest pain, or fever and rigors. These side effects are rarely severe and usually do not last long, but may cause patients anxiety and distress unless they are warned and given appropriate medication. Acute changes in peak expiratory flow rate have also been described, and caution is needed in patients with substantial airway obstruction. A short course of corticosteroids such as prednisolone given for a few days during radiotherapy may be helpful.
Spinal cord damage (radiation myelopathy) was recorded in a few cases in the clinical trials following the use of 17 Gy in 2 fractions and 39 Gy in 13 fractions. Care should therefore be exercised when using these regimens, and steps should be taken to avoid the spinal cord or reduce the dose, especially in patients with a relatively good prognosis and when lower energy radiation (e.g., from cobalt 60) with a less favorable dose distribution is used.
Repeat Radiation
Occasionally, recurrent tumor in the chest causes symptoms in patients who had a good response to palliative radiotherapy. The patient may have had systemic treatment in the meantime, but disease has progressed despite treatment or subsequently after treatment. The clinical question then arises as to whether it is safe and appropriate to consider a further course of palliative radiotherapy. The major risk from repeat radiation is radiation myelitis, possibly leading to paraparesis, if the spinal cord is included in both treatment volumes and the total dose exceeds tolerance. A further risk is radiation pneumonitis if large volumes of the residual normal lung are included in the retreatment fields.
Repeat radiotherapy is never an easy decision and several factors need to be considered:
- •
clinical benefit gained (degree of improvement and duration) with the first treatment and the likelihood of substantial tumor and symptomatic response from repeat radiotherapy
- •
patient’s likely prognosis
- •
risk of radiation myelitis, given the original dose fractionation and the proposed second dose to the spinal cord
- •
volume of lung in the proposed retreatment field, especially if the beams are arranged to avoid the spinal cord.
Inherent in all these considerations is great uncertainty, and, in particular, little is known about the cumulative risk to the spinal cord and whether recovery takes place following initial treatment. However it is reasonable to make some pragmatic judgments about the balance of risk and benefit and to discuss these openly with the patient and his or her relatives. If after being fully informed, the patient is prepared to accept what is probably for most patients with a limited prognosis a quite small risk of myelitis, versus an uncertain but likely symptomatic benefit, then repeat radiotherapy may be appropriate. Clearly, the health-care provider should only proceed with repeat radiotherapy once clear consent processes have been completed.
Brachytherapy
Endobronchial brachytherapy using iridium-192 is an established method of treating tumors in the main bronchi. It involves bronchoscopy and insertion of a small catheter, which is connected to a device that sends the radioactive source to prespecified positions along the tube that correspond to the location of the tumor; the radioactive sources remain in prespecified positions for a few minutes, as needed to deliver the prescribed dose of radiation. The advantage of this technique is that a high dose can be delivered locally to the tumor but it is not effective if there is bulky extraluminal tumor or complete obstruction of the bronchus.
The authors of a Cochrane review summarized the clinical trial evidence and concluded that brachytherapy is less effective than external-beam palliative radiotherapy as first-line treatment. Its use should therefore be restricted to patients who have symptomatic recurrent tumor, which is mainly within the lumen of the bronchus.
Palliative Chemoradiation Therapy
Many patients with symptomatic incurable NSCLC may be candidates for both palliative chemotherapy and radiotherapy; the usual approach is to sequence the therapy, typically starting with palliative radiotherapy for local symptoms, as the expected symptomatic response rates are overall higher than with palliative chemotherapy. This finding has been confirmed in a randomized phase III study of palliative radiotherapy with or without chemotherapy; toxicity was greater and patient outcomes were not better with the addition of concurrent chemotherapy (fluorouracil [5FU]). In 2013, Strøm et al. published the results of a randomized trial that compared chemotherapy alone (cisplatin and vinorelbine) with concomitant chemoradiation in which 45 Gy in 15 fractions was given along with cycle 2, in patients with “poor prognosis” stage III NSCLC. Survival was significantly better in the patients receiving chemoradiation, (1-year survival 53.2% vs. 34.0%; p < 0.01), even those aged over 70 years and with bulky disease, but with increased toxicity. The survival benefit was not, however, seen in those with World Health Organization Performance Status. Although these results are interesting, they come from only one underpowered trial and should be treated with caution. It would certainly not be appropriate to extrapolate them to the management of those with stage IV disease.
Bone Metastases
Bone metastases will develop in as many as 40% of patients with lung cancer at some point. The prevalence of bone metastases has increased because of longer survival related to advances in systemic therapy, but the median survival of 13 weeks in patients with lung cancer with bone metastases is poor in comparison to that of other tumors. Bone metastases are the most common cause of cancer-related pain, and up to 75% of patients experience symptoms that impair their quality of life. As patients with lung cancer and bone metastases have incurable disease, treatment intent is palliative; treatment goals are pain relief, preservation of mobility and function, prevention of skeletal-related events, and optimized quality of life.
Bone metastases can be described as complicated or uncomplicated; complicated generally refers to impending or established pathologic fracture, previous surgery, impending or established spinal cord compression, impending or established nerve-root compression, neuropathic pain, previous radiotherapy, or associated soft-tissue mass. Skeletal-related events are usually defined as pathologic fracture, spinal cord compression, hypercalcemia, or the need for surgery or radiotherapy. Management costs of bone metastases secondary to lung cancer are substantial and primarily driven by treating skeletal-related events.
The selection of the optimal method of palliative radiotherapy depends on symptom burden, extent of disease, life expectancy, performance status, comorbidities, toxicity, risk of skeletal-related events, prior treatment, whether metastases are (un)complicated, and patient wishes and would be best assessed and determined by an interdisciplinary team. Table 41.2 lists the indications and the usual fractionation schedules recommended.
| Indication | Recommended Schedule | Select References |
|---|---|---|
| Uncomplicated bone metastases | 8 Gy/1 fraction | D’Addario 2010 ; Lutz 2011 ; Vassiliou 2009 ; Macbeth 2007 ; Kvale 2007 |
| Impending pathologic fracture, radiotherapy alone Established pathologic fracture, radiotherapy alone Impending/pathologic fracture, postoperative radiotherapy | 20 Gy/5 fractions, 30 Gy/10 fractions, or 40 Gy/15 fractions | Agarawal 2006 ; Kvale 2007 ; Townsend 1994 |
| Neuropathic pain | 8 Gy/1 fraction or 20 Gy/5 fractions | Roos 2005 |
| Associated soft-tissue mass | 20 Gy/5 fractions or 30 Gy/10 fractions | NCCN |
| Hemibody radiation | 6–8 Gy/1 fraction | Salazar 1986 |
| Highly conformal radiation, stereotactic ablative radiotherapy | 20 Gy/1 fraction | Gerszten 2006 |
| Repeat radiation | 8 Gy/1 fraction or 20 Gy/5 fraction | Chow 2013 |
| Impending spinal cord compression, radiotherapy alone | Multifraction | NICE 2008 |
| Established spinal cord compression, radiotherapy alone | 8–30 Gy/1–10 fractions a | Prewett 2010 ; Rades 2010 ; Maranzano 2009 ; NICE 2008 ; Maranzano 2005 |
| Spinal cord compression, postoperative radiotherapy | 20–30 Gy/5–10 fractions | NICE 2008 ; Patchell 2005 |
International Bone Metastases Consensus End Points
Although many phase III trials assessed the palliative benefit of radiotherapy in patients with painful bone metastases, caution is required when interpreting and generalizing the results of older studies because of variable definitions of response, including what constitutes complete and partial relief. The International Bone Metastases Consensus Working Party has defined a uniform set of eligibility criteria, end point measurements, repeat radiation guidelines, and statistical analysis for clinical trials to increase consistency in reporting.
Indications for External-Beam Radiotherapy: Uncomplicated Bone Metastases
External-beam radiotherapy provides durable and timely symptom relief while minimizing toxicity, resource utilization, and the number of visits to the cancer center, but would not be expected to confer a survival benefit. Tumors do not have to be completely eradicated in order to improve symptoms, and therefore doses lower than that required for lesion ablation are used for palliation. Moreover, the treatment of asymptomatic bone metastases may be deferred unless there is a risk of a serious adverse event such as spinal cord compression.
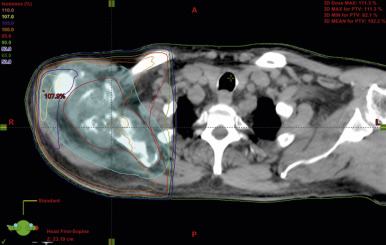
Authors and investigators of more than 20 randomized controlled trials, two systematic reviews, and four meta-analyses have shown that single-fraction palliative external-beam radiotherapy provides equivalent pain relief to multiple fractions in patients with a variety of tumors (up to 25% in lung cancer). The most recent meta-analysis included 25 studies comprising 2818 patients who were randomly assigned to single-fraction radiotherapy and 2799 to multiple-fraction radiotherapy. The overall response rate to single-fraction radiotherapy was 60%, and the complete response rate was 23% (intent-to-treat), which was comparable to the 61% and 24% rates for patients who received multiple-fraction radiotherapy. There were no differences in acute toxicity, pathologic fracture (3%), or spinal cord compression (2% to 3%). None of the meta-analyses separated out treatment effects by histology, and there is no convincing evidence that outcomes differ by primary site. Single-fraction radiotherapy has been repeatedly recommended as the standard of care for uncomplicated bone metastases ( Table 41.2 ) Fig. 41.2 demonstrates a radiotherapy plan using an anterior and posterior beam to deliver 8 Gy in one fraction to a painful right scapular metastasis.
Despite this strong evidence, there has so far been reluctance to adopt single-fraction schedules as standard practice in many countries. In a large prospective study conducted in the United States, 25 in 1574 patients with lung cancer and metastatic disease who received palliative bone radiotherapy, only 6% received single-fraction radiotherapy. Patients who were younger than age 55 years, had had surgery at a metastatic site, or had received chemotherapy were more likely to receive radiotherapy. Patients treated in integrated networks (e.g., health maintenance organizations) received on average 3.4 fewer fractions ( p = 0.001) and 4 Gy less dose ( p = 0.049), although the overall rates of radiotherapy delivery were similar. Suggested reasons for this reticence include country of training, membership affiliation, institutional structure, available pain management teams, wait times for radiotherapy consultation or delivery, reimbursement levels, and departmental policy.
Indications for External-Beam Radiotherapy: Complicated Bone Metastases
Impending Pathologic Fracture
An impending pathologic fracture is defined as a bone metastasis that has a substantial likelihood of fracture under normal physiologic loads. Patients with an impending pathologic fracture may benefit from surgery, radiotherapy, or both, but no randomized trials have reported a comparison of these modalities. Provided that the surrounding bone can support the implanted hardware, prophylactic stabilization reduces pain and avoids the serious consequences of fracture, although postoperative recovery may delay continuation of systemic therapy. Generally, postoperative radiotherapy follows. A proportion of patients will not be candidates for operative intervention or will refuse. In that case, radiotherapy alone can be delivered.
Established Pathologic Fracture
If a pathologic fracture has occurred, surgery with suitable reconstruction is indicated, especially for lower limb bones in patients with a good prognosis. Surgery cannot prolong survival but improves stability, activities of daily living, function, pain, and quality of life. Most guidelines recommend fractionated radiotherapy if surgery is not indicated ( Table 41.2 ), although goals of care and expected lifespan may result in the decision to prescribe single-fraction radiotherapy. In an apparently solitary, histologically confirmed metastasis, especially after a long disease-free interval, doses on the higher end of the spectrum (e.g., 30 Gy in 10 fractions) may be prescribed, although there is no conclusive evidence that this improves local control.
Postoperative Radiotherapy
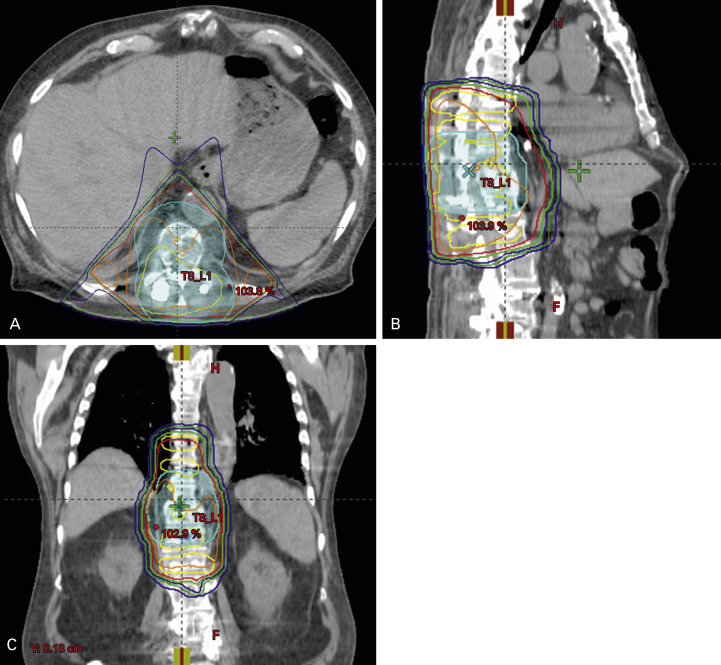
Postoperative radiotherapy is used to promote bone healing by suppressing tumor growth, preventing destabilization of the prosthesis by maintaining the structural integrity of the bone in which the implant is fixed. Postoperative radiotherapy decreases pain, increases the frequency of normal use of the extremity, prevents disease progression, minimizes the need for revision procedures, and reduces the risk of refracture. Following intramedullary nailing, all implanted hardware should be included in the radiation portal to decrease the risk of seeding ( Fig. 41.3 ). Treatment is generally started within 2 to 4 weeks after surgery, once the wound has healed satisfactorily. Although commonly a multiple-fraction schedule is prescribed ( Table 41.2 ), patients with postoperative clinical deterioration may be considered for single-fraction radiotherapy.
Neuropathic Pain
Although neuropathic pain from bone metastases does not usually respond well to standard analgesics, it does respond to radiotherapy. Roos et al. compared 8 Gy in a single fraction with 20 Gy in five fractions for 245 patients with any primary site; 31% had lung cancer. Pain relief was seen in 53% of patients who had single-fraction radiotherapy and in 61% of patients who had multiple-fraction radiotherapy (intent-to-treat) at 2 months. The median time to treatment failure was longer in the fractionated arm (3.7 vs. 2.4 months), but did not reach significance. The authors recommended 20 Gy in five fractions; however, patients with decreased performance status, shorter expected survival, or substantial comorbidities should receive single-fraction radiotherapy ( Table 41.2 ).
Quality of Life
As the main treatment goal of radiotherapy is symptom control, the most appropriate way to assess response (pain, other quality-of-life aspects) is by patient-reported outcomes. Measuring quality of life was recommended for all bone metastases clinical trials by 91% of experts in an international survey. Authors of many recent studies have reported health-related quality of life and functional interference after palliative radiotherapy for bone metastases. In general, patients whose pain improves after radiotherapy also experience substantially improved physical and role function. Improvements extend to associated symptoms, such as insomnia and constipation, and responders describe better emotional functioning, general activity, normal work, and overall quality of life by 2 months. Neither anatomic location nor radiotherapy dose predicts the degree of improvement. Differences in the likelihood of functional response by primary site have been suggested; in one study, 27% of patients with lung cancer had responded by 2 months, compared with 70% of patients with breast and prostate cancer.
Indications for Hemibody External-Beam Radiation
Hemibody radiation is an effective treatment for widespread symptomatic bone metastases, particularly for patients with poor performance status, although it used to be practiced more often in the past. It may be delivered to the upper half (base of skull to iliac crest; 6 Gy in one fraction), lower half (iliac crest to ankles; 8 Gy in one fraction), or middle of the body (diaphragm to pubic symphysis; 6 Gy in one fraction). Hemibody radiation provides pain relief in 70% to 80% of patients, often within 24 to 48 hours, and may decrease future requirements for opioids and local external-beam radiotherapy.
Indications for Stereotactic Radiosurgery
Stereotactic radiosurgery is administered as a radioablative dose to a lesion in a highly conformal manner, typically in a single or a few fractions. It is presently being investigated for certain clinical circumstances, such as repeat treatment of bone metastases near the spinal cord. There are possible advantages with stereotactic radiosurgery:
- •
Smaller volumes of normal tissue radiated
- •
Higher degree of preservation of organ function
- •
Radiobiologic benefits due to the high dose per fraction
- •
Improved local control
However, these advantages may be outweighed by cost, need for specialized equipment, and laborious set-up and treatment. Randomized trials comparing conventional external-beam and stereotactic radiotherapy for bone metastases are ongoing. Gerszten et al. prospectively evaluated outcomes of single-fraction radiosurgery delivered to 87 patients with spinal bone metastases secondary to lung cancer. Of the 87 patients, 70 patients had previous maximum external-beam radiotherapy. The mean tumor dose was 20 Gy in one fraction, delivered over an average of 90 minutes. No patient experienced radiotherapy-related neurologic deficits. A total of 65 of 73 patients were treated primarily for pain and had long-term improvement. Enrollment of fit patients in clinical trials at centers with sufficient experience should be encouraged.
Side Effects of External-Beam Radiotherapy
Acute side effects are generally mild and self-limiting or controllable by conservative measures. Most begin 1 to 2 weeks after treatment, although some, such as nausea, begin within hours. They may not peak until after the end of radiotherapy and usually resolve in 2 to 3 weeks. As radiotherapy is a localized treatment, all the potential side effects except fatigue are site-specific. Late effects, which may appear months or years after treatment, are uncommon, usually permanent, and should be managed by a radiation oncologist. Although the possibility of late toxicity in patients with incurable malignant disease must be considered, many patients will not live long enough to be at risk. Side effects and recommended management strategies have been reviewed.
Pain flare is a common side effect in patients who have undergone external-beam radiotherapy for bone metastases. Pain flare is a short-lived worsening of pain in the treated area, can occur in up to 44% of patients within a week after starting radiotherapy, and lasts for a median of 3 days. It is not clear whether pain flare is more common after single fractionation. In one study, the authors found that patients with lung cancer were less likely to experience pain flare (23%) compared with patients with breast and prostate cancers. In a recently reported double-blind, placebo-controlled randomized trial, dexamethasone 8 mg orally starting on the day of single-fraction radiotherapy and for 4 consecutive days afterward significantly decreased the rate of pain flare with tolerable side effects.
Integration of External-Beam Radiotherapy With Other Modalities
External-Beam Radiotherapy and Minimally Invasive Techniques
Percutaneous vertebroplasty and kyphoplasty are minimally invasive outpatient surgical techniques for restoring stability to lytic spinal bone metastases, as well as improving pain and mobility and preserving quality of life, functional independence, and performance status; when used in the pelvis and elsewhere, the technique is referred to as osteoplasty. These treatments are an option for patients with medical comorbidities, mechanical component to their pain, even if multilevel spinal disease but cannot be used in the setting of a soft-tissue mass. There is no evidence that adding external-beam radiotherapy improves clinical outcomes, although both can decrease pain refractory to other modalities.
External-Beam Radiotherapy and Systemic Therapy
Chemotherapy addresses systemic as well as bone disease. It can improve pain and quality of life and prolong survival in patients with bone metastases, but may be hazardous for heavily pretreated patients with extensive bone marrow disease. Response rates and duration are usually lower than that of radiotherapy, drugs can be expensive, there may be a long interval before onset of relief, and side effects are also systemic. Concurrent radiotherapy and chemotherapy have not been extensively investigated in patients with bone metastases although it is common for patients with bone metastases to receive these modalities sequentially.
Bisphosphonates may prevent or delay skeletal-related events in lung cancer. Because bisphosphonates and external-beam radiotherapy have different dose-limiting toxicities, bisphosphonates could provide background control alongside acute local pain relief provided by radiotherapy. Vassiliou et al. published their experience of concurrent radiotherapy (30–40 Gy) and monthly intravenous ibandronate in 45 evaluable patients with multiple primary sites; 29% had lung cancer. All groups had substantial reductions in pain and opioid requirements, and improved performance status and physical functioning. At 3 months, the complete response rate was 69% and the partial response rate was 31%. Radiographic bone density and lesion healing were substantially increased at all time points. No patient required repeat treatment, and only one pathologic fracture occurred in a patient with lung cancer. Other studies evaluating the combination have been reviewed by Vassiliou et al.; no randomized data are available. External-beam radiotherapy and concurrent bisphosphonates have been recommended by one guideline. They are also a consideration where radiotherapy is contraindicated.
Denosumab is a fully human monoclonal antibody, which specifically targets the receptor activator of nuclear factor kappa B ligand, an osteoclast regulatory factor. It may delay the onset of bone metastases and treat established bone destruction. In patients with bone metastases due to NSCLC, denosumab may increase median overall survival compared with zoledronic acid (9.5 vs. 8 months). Denosumab is recommended as an option for preventing skeletal-related events in adults with bone metastases from solid tumors if bisphosphonates would otherwise be prescribed or after bisphosphonates have become ineffective. However, there is no high-quality evidence available on combined denosumab and radiotherapy.
Repeat Radiotherapy
Repeat radiotherapy may be considered when other treatments are either ineffective or not indicated. Rates of repeat treatment in the most recent meta-analysis were 20% after 8 Gy in one fraction compared with 8% after multiple-fraction radiotherapy ( p < 0.00001). One reason more patients receive repeat treatment after SF radiation is because they can; many radiation oncologists are reluctant to deliver repeat treatment after ≥30 Gy, particularly if the spinal cord is in the volume. An intergroup study randomly assigned 850 patients who had received previous radiotherapy and had painful bone metastases to receive radiotherapy at 8 Gy in one fraction or 20 Gy in five fractions. The intention-to-treat response rate at 2 months after 8 Gy (28%) was no lower than that after 20 Gy (32%); by per-protocol analysis, rates were 45% and 51%, respectively. There were no differences in rates of pathologic fracture, spinal cord compression, or quality of life, but adverse events at day 14 were substantially worse after multiple fractions. Studies that report time to maximum response after external-beam radiotherapy have found it commonly takes 4 to 6 weeks. Therefore, repeat treatment should be delayed until this time, which also allows response to the first course to be assessed and pain flare to have resolved.
Spinal Cord Compression
Spinal cord compression will develop in 5% of all patients with cancer, and lung cancer is ultimately diagnosed in 15% to 30% of patients with spinal cord compression as their presenting event. The median survival from diagnosis of spinal cord compression due to lung cancer is poor: it is less than 2 months in one study. Urgent magnetic resonance imaging (MRI) of the entire spine should be undertaken on the least suspicion of neurologic symptoms or signs, especially in a patient with known vertebral metastases.
Definitive treatment (surgery or radiotherapy) within 24 hours is recommended to optimize symptom control, preserve neurologic function and ambulation, decrease tumor bulk, and maximize quality of life. The spinal service should be consulted for an opinion if a tissue diagnosis is required; there is a solitary level of compression; there is spinal instability or bony fragments within the spinal canal; the patient cannot have radiation (e.g., because of previous radiation at that level); neurologic deterioration occurs during or after maximal dose radiotherapy; there is radiotherapy-resistant histology; there is rapid symptom progression; or there is acute onset paraplegia.
In general, factors associated with a better prognosis include longer time to development of motor deficits, ambulatory ability before and after therapy, radiotherapy-sensitive histology, and a single site of compression, treated in a timely fashion.
Best supportive care including opioids, venous thromboembolism prophylaxis, and rehabilitation are reviewed in a 2008 United Kingdom guideline. There is good evidence that corticosteroids are effective. Unless contraindicated, all patients should receive a loading dose of 10 mg to 16 mg of dexamethasone, followed by 16 mg daily in divided doses thereafter. After radiotherapy, the dose can be tapered. If neurologic function deteriorates, the dose should be increased temporarily.
Impending Spinal Cord Compression
Retrospective studies suggest that radiotherapy may preserve neurologic function in patients with spinal metastases with radiographic signs that suggest the spinal cord is at risk. In the absence of good evidence on dose, fractionated radiotherapy should probably be offered ( Table 41.1 ).
Established Spinal Cord Compression
Surgery and postoperative radiotherapy is superior to radiotherapy alone for symptomatic patients with an expected survival of more than 3 months and a single level of compression, resulting in significantly better ambulation rates, retention of ambulation and continence, maintenance of functional and motor scores, and lower doses of steroids and analgesics. This combined-modality approach has therefore been recommended for spinal cord compression or spinal instability (compression fracture, dislocation, or retropulsed bone fragments), in patients with sufficient performance status and life expectancy, by many international bodies. Because patients with SCLC often have a short lifespan, surgery is less often considered. If surgery is not indicated or declined, radiotherapy alone should be offered, which is still the most common treatment for epidural spinal cord compression. The most appropriate radiotherapy schedule is still being debated ( Table 41.1 ). This debate is likely due to the scarcity of good data exploring dose schedules in common use. Patients with spinal cord compression were excluded from most clinical trials investigating radiotherapy for uncomplicated bone metastases. Two randomized Italian phase III trials investigated short-course and single-fraction treatment in patients with poor prognosis. No significant differences were found for relief of back pain, ability to walk after radiotherapy, bladder function, duration of motor improvement, toxicity, or survival between short-course regimens. There were no differences between short-course and single-fraction radiotherapy except for median duration of response, which was longer for 8 Gy in two fractions. The authors of a series of retrospective studies from a large multicenter database have reported similar immediate functional and neurologic outcomes and survival with short- and longer-course radiotherapy. However, longer-course radiotherapy is probably associated with better local control and less in-field recurrence. Although a patient with a poor prognosis who is paraplegic will not benefit from radiotherapy in terms of neurologic status, delivery of external-beam radiotherapy for pain control should be considered. Postoperative radiotherapy should be offered to all patients with a satisfactory surgical outcome once the wound has healed.
Repeat Radiotherapy for Spinal Cord Compression
Recurrent spinal cord compression in a previously radiated region may be treated with further radiotherapy, provided the patient responded well to the previous course and the interval has been greater than 3 months. Highly conformal radiotherapy techniques such as stereotactic radiosurgery provide a method of salvage treatment in patients with limited metastases and good performance status and can be considered to reduce the cumulative spinal cord dose. Although stereotactic radiosurgery could be undertaken for patients who have recurrent pain at a previously radiated spinal segment, it cannot be used in an emergency setting such as frank spinal cord compression. Surgical decompression could be undertaken in the setting of maximal previous radiotherapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree