For older patients with multimorbidity and a new serious problem, as described above, short-, medium-, and long-term goals now may be achievable only over a few weeks or months.
6.2.1 Trajectories of Decline
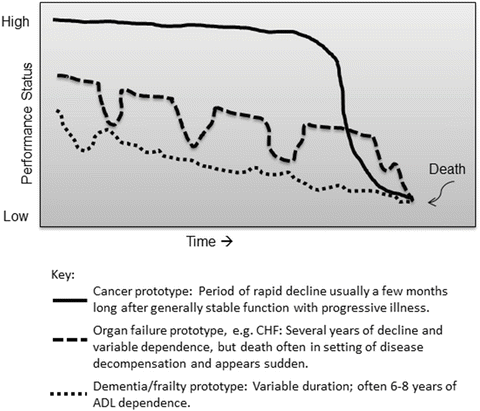
Four prototypic healthcare trajectories for serious illness have been described in the literature: [5, 6] (1) sudden death; (2) death following a disease of progressive, linear decline (e.g., non-treatable cancer); (3) death following an illness with intermittent, acute exacerbations, or a “saw-toothed” functional decline (e.g., congestive heart failure or COPD); and (4) death from gradual progressive functional decline (e.g., neuromuscular disease or dementia). The three patterns of decline are shown in Fig. 6.2. In these patterns, the patient typically has been living in a state of variable, but limited, functional reserve often with dependence in activities of daily living (ADLs) for months or years prior to death. These situations, especially with a superimposed new illness or injury, require complex judgments by clinicians.
6.3 The Specialty of Palliative Care Medicine and Its Interface with Other Programs and Specialties
The World Health Organization (WHO ) defines palliative care as healthcare that “Improves the quality of life of patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering.” “It affirms life, and regards dying as a normal process; it intends neither to hasten nor postpone death.” [7] Palliative care focuses not only on the patient, but also on his or her supporters, as all are profoundly impacted: physically, emotionally, socially, and spiritually. While the terms, “palliative care” and “palliativemedicine ” are often used inter-changeably, the broader term “palliative care” is preferred when referring to the multidisciplinary services including interdisciplinary teams and programs aimed at maintaining hope, preserving dignity and autonomy, and improving quality of life (QOL) for patients and families. “Palliative medicine” is a phrase reserved for the portion of a team who are the medical providers only [8]. Palliative support is appropriate at any stage of illness—from diagnosis onward—and can be combined with treatments aimed at disease modification or cure. Team assistance and recommendations differ depending on the stage of illness and the preferences of the patient [9]. For example, while the hospitalized patient’s goal is focused on disease cure or prolongation of life soon after learning of a new serious diagnosis, the palliative team may assist in building rapport with the patient and family and assisting with the practical burdens of illness.
As serious illness evolves, patient goals evolve, often shifting away from attempts at disease modification or cure as treatment options diminish or become more burdensome. During the evolution away from curative or disease-modifying treatment, the options for palliative treatments increase. Patients and families may focus on other goals such as being able to return home, improving physical comfort, alleviating spiritual distress, and other means of maximizing quality of life. Over time, the role of the palliative team will also change, likely assisting with increased symptom management . The palliative team may provide continuity of care and can assist in creating seamless transitions to home or other settings for post-acute care. Hospice services at home, or in an inpatient setting maybe a vital option to help the patient leave the hospital, or prevent admission.
6.4 Hospice Versus Palliative Care (Table 6.1)
Table 6.1
Palliative care vs. hospice
Palliative care | Hospice | |
|---|---|---|
Primary goal | – Assist patient to achieve goals – Improve quality of life – Alleviate suffering | – Improve quality of life – Relieve suffering |
Recipients | – Anyone with a serious illness | – Patients whose physicians certify a life limiting illness and a prognosis of 6 months (or less) if illness runs usual course and – Patients who elect their “hospice benefit” (e.g., insurance) |
Providers | – Interdisciplinary Team may include MD, APN, SW, Chaplain, and other staff | – Interdisciplinary Team must include MD, RN, SW, Chaplain, volunteer, and a bereavement specialist. |
Time frame | – Can be initiated at any time from diagnosis onward – Indefinite access | – Life expectancy of less than 6 months. – Services typically continue through death, but may be discontinued if patient’s condition improves and life expectancy exceeds 6 months, or if patient elects to resume disease-modifying treatments. |
Special benefits | – Treatments aimed at cure or disease-modification may be continued | – Provides and pays for medications, durable medical equipment related to the hospice diagnosis. – Volunteers provide some additional services. |
Location | – Mostly hospitals, oncology practices, nursing homes, and group practices. | – Mostly at home. – Widely available. |
Challenges | – Not widely available in the community setting. | – Typically, hospice benefit does not cover disease-modifying therapies such as chemotherapy, IV antibiotics, transfusions, etc. – New models of “concurrent care” (e.g., hospice and some chemotherapy) in some areas, especially if commercial insurance underwrites treatment. – Most hospices require caregiver in the home. – Personal care aide hours very limited. |
Payment | – Professional fees are reimbursed by medical insurance, e.g., MD/NP fees covered by Medicare Part B. – Support from other programs (e.g., hospital, oncology practice) – No payment mechanism from Medicare for non-medical providers e.g., chaplain, personal care aides. | – Medicare Hospice Benefit or as defined by commercial payers. |
While palliative care includes hospice care it is not limited to this. Hospice services are limited to those whose life expectancy is estimated to be less than 6 months, and focuses on end-of-life care. Palliative care providers care for patients at any point in the trajectory of a serious disease, regardless of prognosis [10].
In the USA, hospice is an integrated bundle of services that is covered by medical insurance. Hospice provides services, durable medical equipment and medications related to the terminal diagnosis (not to other co-morbidities). The care is provided by an interdisciplinary team. The Medicare Hospice Benefit requires each hospice to provide nurse case management services, access to physician services, chaplaincy, social work, and volunteer support. Bereavement counseling for 13 months after a patient’s death is also offered. To enroll a patient in hospice, two physicians must certify they believe the patient has a life expectancy of 6 months or less. Patients are reassessed for hospice service eligibility at regular intervals, and services may extend beyond 6 months if a patient’s condition is continuing to decline. However, if the patient’s condition stabilizes, regulations dictate consideration of discharging the patient from hospice. Patients can revoke the hospice benefit at any time, such as if they are hospitalized. A re-evaluation for eligibility is required before enrolling in hospice again.
Hospice services are provided in the location the patient defines as “home” and, for example, may be engaged in a patient’s private home, a nursing home, group home, or assisted living facility. The Medicare Hospice Benefit is commonly used and serves as the prototype for most other insurers.
6.5 Palliative Medicine vs. Geriatric Medicine
Palliative medicine and geriatric medicine share many common features. Both rely on interdisciplinary teams to provide care. Geriatric medicine emphasizes the importance of comprehensive assessment to optimize a patient’s function. Palliative medicine focuses primarily on optimizing quality of life through alleviation of adverse symptoms, helping patients and families identify goals of care, and supporting effective emotional coping for patients and families. Both specialties encompass the care of older adults near the end of life and overlap in the care of frail elders. Both palliative and geriatric medicine teams have expertise in evaluating a patient’s expected course, communication with patients and families to decide goals of care, develop advance care plans, and providing care throughout the course of the illness. Palliative providers have additional training and expertise in determining life expectancy, symptom control at end of life, and management of special populations of patients, such as those in the intensive care unit (ICU), patients with oncologic emergencies, nearing death with heart failure, human immunodeficiency virus (HIV), amyotrophic lateral sclerosis (ALS) and other degenerative neurological diseases, COPD, and chronic incapacitation from trauma. Geriatricians more typically have expertise caring for patients needing palliative care in nursing home, assisted living and home care venues. Both geriatric and palliative medicine specialists identify patient goals of care and address the comprehensive elements of function and well-being.
6.6 Evidence Base for Palliative Care
The Center to Advance Palliative Care (CAPC ) (www.capc.org) has developed a venue for sharing expertise, tools, and resources in order to promote the integration of palliative care into every clinical setting serving those with serious illness. When palliative care is integrated into the care of patients in the ICU, research shows increased family satisfaction and comprehension; decreased family anxiety, depression and post-traumatic stress disorder; decreased conflict over goals of care; decreased time from recognition of poor prognosis to comfort-focused goals; increased symptom assessment; increased patient comfort; decreased use of non-beneficial treatments, and decreased ICU and hospital length of stay.
In the ED, there is evidence that proactive palliative care results in decreased hospitalizations, decreased in-hospital deaths, and increased use of hospice care. While the sites of care for palliative medicine consultation have historically been centered in the hospital setting, palliative services are moving to other places of need including outpatient clinics, assisted living environments, and nursing homes. Various models for outpatient palliative care services have been implemented. In one randomized trial, early palliative care for patients with non-small cell lung cancer in the outpatient setting resulted in improvements in both quality of life and mood. Participants also had less aggressive medical care at the end of life and longer survival [11].
The Institute of Medicine (IOM ) report “Dying in America” described the state of end-of-life care in the USA [12]. This 2014 report concluded that improved medical and social supports to both patients and family could enhance quality of life while reducing costs. However, additional research suggested that many studies do not include adequate numbers of seniors [13]. “Geriatric Palliative Care” has been proposed as an “intersection subspecialty” for seniors. The cases presented early in the chapter exemplify this point and highlight the unique complexity present in each patient. Anna requires chronic management of COPD and other co-morbidities. Her acute problem is likely to worsen her other conditions and lead to more debility. Also, she is at high risk for a delirium, which if not prevented will worsen markedly her prognosis [14, 15]. Geriatric palliative care also must carefully address the needs of caregivers who are typically daughters or a senior partner often already overwhelmed with the care of supporting their children, grandchildren or meeting their own care needs. Such situations often preclude home hospice [16–18]. Palliative care planning, therefore, must consider all involved with helping the patient if an effective plan is to be developed [13].
6.7 Skilled Home Health vs. Home Hospice
Home health agencies offer many of the same services as home hospice: nurse case management, durable medical equipment, medication management, and social work assessment. Both skilled home health and hospice are provided by most insurers. The primary goal of hospice services is to improve quality of life through the end of life and this service continues through death (unless the patient is discharged from hospice). The goal of skilled home health services, however, is to resolve a medical or surgical problem (e.g., a wound) or functional loss from an illness or injury (e.g., therapy after a stroke). Services are short term and stop once the patient maximally improves. Hospice agencies provide 24-h telephone access to assist caregivers in managing urgent issues with the goal of caring for the patient effectively at home and avoiding hospital care. On the other hand, most skilled home health agencies do not have a 24 h on-call program and urgent needs must be provided by the primary care provider.
6.8 Palliative Care for all Clinicians
Basic palliative medicine attitudes and knowledge are appropriate for all clinicians caring for patients with serious illness [19, 20]. Such training and expertise in so-called primary palliative care has been endorsed by the 2014 IOM report [19, 21]. These proposed primary palliative skills needed by all clinicians include:
Ability to elicit patient-centered goals of care
Ability to develop and convey prognostic information and treatment options
Assessment of pain and other physiologic and psychological symptoms
Assessment of spiritual or social/practical burdens of illness
Coordination of care for a safe transition to the next level of services
A study of over 1000 patients over age 80 years with serious illness showed the unique issues in this population compared to younger adults and children [22]. These older patients had a higher prevalence dementia, reduced prevalence of cancer, fewer recommendations for symptom management, and more questions concerning decision-making capacity, more issues related to withholding/withdrawing life-sustaining treatments and took more time to complete. These differences were substantiated in a slightly younger group, which showed that 70 % of patients older than 60 years lacked decision-making capacity at the time health care decisions were made [23]. In a third study, prior advance care planning conversations have been shown to reduce the emotional distress of surrogate decision makers of ICU patients [24]. These three studies point out the complexity of palliative care for seniors and the importance of advance care planning.
6.9 Communication and Shared Decision Making
Even today, many clinicians have received limited training in communication, estimating life expectancy, or breaking bad news [4, 25, 26]. As a result, many clinicians feel unprepared to help patients needing palliative and end-of-life care [4]. Patients with serious illnesses and their families or other caregivers desire and need clear and honest information [27, 28] from clinicians in order to wisely plan all aspects of their future: who will provide care, where will it be provided, what are the goals of care, what will become of their finances, what about their employment, and how do they think about dying. A stepwise approach of a compassionate explanation of the development of their current situation and the current options is best in hosting a family meeting and/or breaking bad news. Doing this thoughtfully and openly, with a wise sense of the course of the illness, and with sensitivity and empathy, decreases stress, confusion, false hopes, and anger for all. Such a conversation allows for the development of a satisfactory and shared decision that will help address all aspects of care including venue transitions and potential self-pay concerns [4, 27, 29].
A review of communication strategies with patients who have serious illness provides best practices and advocates for shared decision making using the patient-centered care model [27]. These strategies mirror the AGS [1] decision-making paradigm: (1) assess the patient’s and family’s understanding of the disease state and prognosis; (2) ascertain patient preference about information sharing and decision making; (3) involve family as guided by the patient; (4) discuss patient priorities, fears and thoughts about quality of life and function; (5) explore tradeoffs in quality of life, as patients often have goals more important than longevity, and (6) convey as accurate prognostic information as possible.
One approach to breaking bad news and negotiating a patient-centered care plan uses the mnemonic SPIKES , for Setting up the encounter, asking the patient their Perception of the medical situation, requesting an Invitation to share prognostic information, provide Knowledge, Empathize with emotion and Summarize and Strategize for next steps [4]. An example of how this approach could be employed to assist in a conversation with Bob, a patient story early in the chapter, is shown in Table 6.2.
Table 6.2
The SPIKES model employed in Bob’s case
Context | Words to consider: | ||
|---|---|---|---|
S | Setup | • Find a private space, if in double room, draw curtain • Ensure patient comfort • Ensure uninterrupted time o Turn off pager, phone o Ensure presence of significant others o Family/friends o Medical team | • Use non-verbal actions which show commitment to the patient and value to the dialogue: o Sit down o Ensure patient’s physical comfort o Attentive, open posture |
P | Perception | • Assess patient/family’s understanding of what is happening • Note vocabulary used by patient • Share decision making on meeting agenda | • “What is your understanding thus far about your jaundice, and what did you hope to learn today?” • “To make sure we are starting in same place, can you tell me what you understand about your yellow eyes, and what I can help you understand?” |
I | Invitation | • Obtain patient’s invitation to discuss details of illness; “ask” • Key with cross-cultural dialogues • Key with prognostic information | • “Are you the type of person who likes to know all the details about what is going on, or would you prefer I speak with your son?” • “How much information would you like to know about the future? About your diagnosis?” • “Would discussing prognosis be helpful to you?” |
K | Knowledge | • Give a warning shot • Provide information info in small chunks and check for understanding • Avoid technical words and mirror patient’s word choice o If patient says “growth,” you say “growth” | • “I have your test results, and I have some bad news…” • E.g., “It appears as if we are unable to provide surgery due to the position of the cancer.” • “What questions do you have? Is there anything I can help clarify?” |
E | Empathize | • Empathize and explore the emotions expressed by the patient • Acknowledge emotion • Normalize feelings • Explore • Use “I wish” statements | • “I know these are not the results we wanted.” • “Tell me what the hardest part of this is for you?” • “I am upset! Why did that surgeon examine my dad! I thought that meant he was going to have surgery!” |
S | Summarize and Strategize | • Summarize what has been said • Clear plan for next steps and follow-up | • “Are there any last items I can help clarify?” • “Let’s meet again at noon tomorrow to continue this discussion, after the medical oncologist has visited.” • “I want to make sure I have clearly explained your dad’s situation. Can you give me your understanding of what is ahead?” |
Contrary to the belief of some clinicians, patients are not harmed by discussions of end-of-life issues or goals of care planning [27]. Rather, patients and families wish to control the amount and timing of information they receive, especially when it relates to the prognosis of an illness(s) [28]. Commonly, patients and families need for information diverges as an illness progresses: family members typically want more detailed information and patients less.
The wise clinician must acknowledge and responding to patient and family emotions [30]. Unaddressed emotions can interfere with the ability to process and retain information, and may impair decision-making ability. Promptly responding to emotion—either verbally or non-verbally—legitimizes the feelings expressed and conveys openness on behalf of the clinician to discuss concerns fully and as they arise in the future [27, 30].
Finally, sensory and cognitive impairments are common in older adults, and may affect their ability to understand information presented verbally or visually (Table 6.3) [31, 32].
Table 6.3
Communication challenges and strategies with older adults
Potential challenges | Strategies |
|---|---|
Low vision | – Ask and assess if low vision is present. – Adjust communication materials to avoid visual prompts. – Use “teach back” technique to assure understanding. |
Hearing loss | – Ask and assess if able to hear adequately. – Establish optimal environment to promote communication, e.g., quiet, well lit room, seated directly in front of patient, minimize distractions. – Provide “pocket talker” or similar hearing augmentation tools. – Use “teach back” technique. |
Low health literacy | – Reduce complexity of communication. – Avoid jargon and technical terms. – Try to use their words when possible. – Reduce the density of communication, no more than three concepts per encounter. – Use “teach back” technique. |
Memory impairment | – Assess cognitive function by history-taking, chart review, or cognitive screen. – Identify family or health care proxies to participate. |
Reduced concentration | – Optimize environmental factors to promote concentration. – Assess ability to concentrate and receive information. – Identify family or health care proxies to participate. |
Cultural influences | – Recognize language and cultural barriers to communication. – Ask about communication preferences, – Ask about individual values and cultural backgrounds and seek to understand and integrate into care. – Use “teach back” technique for patient and family/health care proxies. |
Role expectations | – Ask what and to whom information should be disclosed. – Ask about preferences for decision-making strategies. |
6.10 Symptom Relief in Serious Illness and EOL
Palliative assessment and treatment must incorporate considerations of the distinct pattern and severity of a patient’s co-morbidities. Persons with dementia have unique needs in palliation. Cognitive impairment decreases a patient’s ability to articulate complaints of distressing symptoms and to understand the disease process and develop and voice goals of care. Therefore, recognizing nonverbal cues are especially important to effective management. In situations of cognitive impairment, behavioral interventions (e.g., a compassionate and appropriate touch) are helpful. In the hospital, seniors are affected greatly by their environment. Noise, lighting, multiple visitors or providers at once, irritating interventions (e.g., IVs, oxygen, or catheters) can worsen agitation and discomfort, and these must be routinely assessed and modified. Seniors with chronic conditions and/or frailty often experience severe and distressing symptoms, such as limited activity, fatigue and physical discomfort or pain, which must be addressed [33, 34]. Because of co-morbidities and age-related physiological loses, drug management is complex in older patients needing palliative care. Chapter 5 provides an in-depth consideration of these issues. Of special consideration in palliative care two cardinal points should be made: use oral medications whenever possible, and when significant pain is present, avoid long-acting opioids, until the appropriate drug and formulation using short acting agents has been established.
6.10.1 Symptom Assessment
A structured review of patients’ goals and their current distressing symptoms needing attention is key to successful treatment. Such a review will need to be repeated frequently. For example, symptom management for a patient with only hours or days to live is likely to focus solely on comfort measures without concern of over sedation. When life expectancy is measured in weeks to months, concern for adverse effects of medications typically remains prominent. While cognitive impairment can make symptom assessment and management more challenging, nursing home residents with mild-to-moderate cognitive impairment have been shown to have self-reports of pain as valid as those without cognitive impairment [35]. Tools for symptom assessment include the easy to use Edmonton Symptom Assessment Scale [36]. This measures ten levels of distress for ten common symptoms including pain, fatigue, nausea, anorexia, dyspnea, and several affective features.
Patients with cognitive impairment have difficulty recalling previous symptoms making their comparison of interventions difficult. In this situation, the clinician must rely on changes in function, behavior or mood, as these may mirror improvement or deterioration of symptoms [37]. Several validated instruments are available to use in patients who are unable to communicate because of aphasia or intubation [38–40].
6.10.2 Approach to Management (Table 6.4)
Table 6.4
Palliative treatments for symptoms
Symptom | Special features | Treatments | Special considerations |
|---|---|---|---|
Somatic pain | • Opioids first line for cancer pain. | • Morphine oral/IV/SQ • Oxycodone oral • Hydromorphone oral/IV/SQ • Fentanyl transdermal/IV | • Always start bowel medications at the same time as opioids to prevent constipation. • Start with half the opioid dose recommended for younger adults • Fentanyl and oxycodone are safer than morphine in those with renal impairment. |
Neuropathic pain | • Adjuvants or co-analgesics helpful in addition to opioids. | • Gabapentin— may cause dizziness. • Pregabalin—25–50 bid in debilitated patients • Tricyclic antidepressants (TCA) • Carbamazepine • Selective serotonin reuptake inhibitors (SSRI) • Mixed reuptake inhibitors (SNRI) • Topical lidocaine patches. • Ketamine IV/PO (limited data in older adult) • Methadone | • Start with gabapentin 100 mg at bedtime. Increase weekly, as tolerated. • If stopping gabapentin or pregabalin, taper over a week to avoid seizures. • Gabapentin and pregabalin both require renal adjustment • Methadone is dangerous due to unpredictable metabolism and interaction with many other medications. May prolong QTc. For use by experienced prescribers only. |
Dyspnea | • If bronchospasm present, give bronchodilators. • If volume overloaded, give furosemide 40 mg PO/IV one dose. • If oxygen sats <90, give oxygen 2 l/min. • Low dose opioids relieve dyspnea | • Albuterol 2 inhalations every 4 h prn or 3 ml nebulizations every 2 h prn. • Morphine 5 mg PO every 2 h prn or 2 mg SQ/IV every hour prn. | • Monitor respirations. • Consider non-pharmacologic options including fans, relaxation, CPAP, BiPAP, physical comfort measures. |
Anxiety | • Investigate causes. | • Non-pharmacologic treatments first (empathic listening, psychotherapy, integrative therapies such as music, relaxation mindfulness, Reiki, massage) • SSRI—may take weeks for full effect. • Gabapentin or Trazodone. • Short acting benzodiazepine e.g., lorazepam. • Long-acting benzodiazepine if chronic (e.g., clonazepam). | |
Delirium | • Common in older adults, especially those with low vision and hearing and cognitive impairment. Seek underlying cause. Anticholinergic medications likely to precipitate or worsen (e.g., diphenhydramine). | • Haloperidol 0.5 mg PO/IV/SQ every 4 h as needed. Increase by 1 mg every hour until desired effect. Maximum daily dose 20 mg. | • Consider QTc monitoring at higher doses. |
Constipation | • Fecal impaction more common in older adults. Can lead to urinary retention. | • Senna • Docusate • Add milk of magnesia concentrate if supported by renal function 10 mg PO daily or bisacodyl 10 mg PO/PR every day.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|