Palliation of Visceral Recurrences and Metastases and Treatment of Oligometastatic Disease
The 5-year survival and overall cure rates for many cancers have significantly improved over the past 40 years. Unfortunately, there is still a substantial minority of patients who develop recurrent or metastatic disease. The most common sites of metastatic disease are bones, brain, lungs, and liver. In addition, there are other visceral sites of recurrence or metastatic disease that may cause significant symptoms. Palliative radiation therapy for treatment of bone and brain metastases is well established, with multiple randomized prospective trials evaluating best treatment practices. However, there is less information on treatment of visceral metastases, although radiation oncologists recognize that radiation therapy is frequently effective at palliating symptoms from visceral metastases.
The specific goals of treatment will depend on the symptoms, treatment site, functional status of the patient, and other patient-specific factors. The indications for treatment may include pain, visceral obstruction, bleeding, or other symptoms that negatively impact the quality of life. In general, this treatment is given with the goal of improving the quality of life, not the quantity of life. For some disease sites, this can be done with a very short course, low-dose treatment. For other sites, this will require higher doses given to control the local disease.
Some visceral metastases will cause pain. For example, liver or adrenal metastases may cause significant pain. Splenic metastases from hematologic metastases may cause abdominal pain, early satiety, and abdominal distention. In contrast, pulmonary metastases rarely cause pain. These lesions may cause airway obstruction or hemoptysis. Metastatic lesions in the gastrointestinal system may cause bleeding or obstructive symptoms (esophageal obstruction, gastric outlet obstruction, gastric bleeding, biliary obstruction, rectal obstruction). In addition to rectal obstruction, pelvic masses may cause bleeding (vaginal, bladder, or rectal), ureteral obstruction, or pain from involvement of the sacrum or sacral nerves.
There has been a revolution in the understanding of the metastatic process in the past decade. Traditionally in solid tumor biology, cancer cells are thought to undergo some genetic change that potentiates them to grow into tumors and cause daughter cells to spread through the lymphatic or circulatory system to lymph nodes and distant sites. However, recent understanding of the epithelial–mesenchymal transition demonstrates that cancer cells develop embryonic stem cell–like properties that allow them to disseminate to distant sites. The microenvironment surrounding the primary tumor and metastatic sites can regulate the metastatic process. In addition, metastasis occurs much earlier than clinical detection of lymphadenopathy and radiographic or symptomatic evidence of the development of distant visceral disease.1 Treatment of visceral metastases in the oligometastatic setting may allow for improved palliation and may also improve the overall survival in carefully selected patients.
 MALIGNANT LOWER AIRWAY OBSTRUCTION
MALIGNANT LOWER AIRWAY OBSTRUCTION
The lower airway is generally defined as being below the first tracheal ring. Upper airway obstructions can be emergently treated with a tracheostomy. The lower airway can become compromised from either primary or metastatic malignancy. Patients may have intrinsic compression caused by an endobronchial tumor or extrinsic airway compression by a tumor mass in the lung parenchyma or bulky lymphadenopathy during the course of their illness. Clinical symptoms, bronchoscopy, and radiographic imaging are used to determine the extent of luminal obstruction. It is important to determine whether the airway obstruction is endobronchial or from external compression of the airway in order to select the most appropriate treatment. For intrinsic compression of a tumor mass growing inside the bronchial lumen, interventional bronchoscopy techniques offer immediate benefit in many patients. These interventional techniques can result in relief of dyspnea, decreased risk of postobstructive pneumonia, and improved functional status.2–4 Either laser resection of tumor or endobronchial stent placement, along with radiation therapy, is effective in maintaining the patency of the lower airway.5,6 The additional treatment of the airway can be given with either external-beam radiation therapy, endobronchial brachytherapy, or photodynamic therapy. Brachytherapy is typically the best option in patients who have recurred after prior external-beam radiation therapy. For endobronchial therapy, the recommended doses are 7.5 Gy é 3 or 10 Gy é 2.7
External-beam radiation therapy is typically used for treatment of airway compromise caused by extrinsic compression of a bronchus. Endobronchial brachytherapy typically does not improve symptoms when there is external compression of the airways.8,9 The highest doses from brachytherapy will be given to the (relatively) normal mucosa of the airway, with a much lower dose to the extrinsic tumor that is causing the compression.
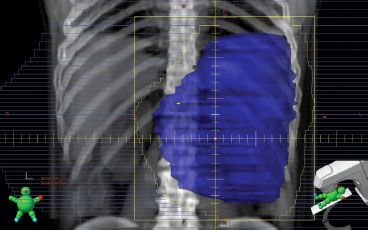
There is no standard fractionation scheme or total dose for palliative external-beam treatment for lung cancer. A review by Fairchild et al.10 found the best results, in terms of palliation of symptoms, with relatively high doses of radiation therapy: 3 Gy per fraction to a total of 30 to 45 Gy, or 2 Gy per fraction to 50 to 60 Gy. The trade-off is that there is a higher rate of acute esophagitis with these higher doses. The American Society for Radiation Oncology clinical practice guideline consensus statement recommends doses equivalent to 30 Gy in 10 fractions or greater.11 For patients with a poor performance status or who have difficulty traveling for multiple treatments, shorter fractionation schemes such as 20 Gy in 5 fractions can be used. Very short regimens (17 Gy in 2 fractions or 10 Gy in 1 fraction) may be useful in certain situations; however, there are reports of radiation myelopathy with these regimens (17 Gy in 2 fractions).11 Figure 95.1 demonstrates an example of a digitally reconstructed radiograph of a palliative lung treatment field. The major acute side effect from this treatment is dysphagia, but careful attention should be made to limit normal lung tissue.
In most patients who receive radiation therapy for palliation of pulmonary symptoms, there is no benefit to the addition of chemotherapy. Combined chemotherapy and radiation therapy may improve response rates slightly, but with significantly greater acute toxicity.11
FIGURE 95.1. Digitally reconstructed images from computed tomography simulation, showing axial, sagittal, coronal, and three-dimensional representations of a palliative lung treatment field. This treatment field is in a patient with hemoptysis from central airway involvement as well as superior vena caval compression.
 LIVER METASTASES
LIVER METASTASES
The liver is a common metastatic site. It is the most frequent site of distant metastatic disease from gastrointestinal tumors, especially colorectal, but also including esophageal, stomach, and pancreatic cancers. The liver is also a frequent site of metastases from lung cancer, breast cancer, and melanoma. A patient with liver metastases may have symptoms of anorexia or early satiety, weight loss, nausea, right upper quadrant or epigastric pain, jaundice, and fever.
For patients with few metastatic lesions in the liver and no evidence of extrahepatic metastases, 12% to 36% may be cured with surgical resection.12 The risk factors associated with the best outcome are clear resection margins, low levels of carcinoembryonic antigen, a single metastatic deposit, metachronous presentation of the liver metastasis, and node-negative disease (with the original primary).12 There are multiple other treatments that can be used for patients with one or a few hepatic metastases, including radiofrequency ablation, microwave coagulation therapy, transarterial chemoembolization, and stereotactic body radiotherapy.13
Most patients with liver metastases are found to have multiple lesions, extrahepatic disease, or other risk factors that make them unsuitable for these surgical or interventional procedures. It is common for these patients to undergo multiple courses of systemic therapy prior to being seen and evaluated by a radiation oncologist. For many years, the only viable chemotherapy option for metastatic gastrointestinal tumors was 5-fluorouracil, often given in combination with leucovorin. More recently, multiple drug regimens including oxaliplatin or irinotecan have significantly improved the response rates and increased the median duration of survival. Targeted agents such as cetuximab or bevacizumab may improve the response rates even further. These new combinations have changed the goal of treatment from palliation to prolonging survival; with this new paradigm, the multiple drug regimens are given as neoadjuvant therapy with the goal of downsizing tumor bulk in order to facilitate a resection.14 Hepatic arterial infusion of chemotherapy gives higher local concentrations of the chemotherapy agents and also gives higher response rates. A meta-analysis of hepatic arterial infusion chemotherapy for liver metastases demonstrated a significantly higher response rate but showed no improvement in survival duration.15
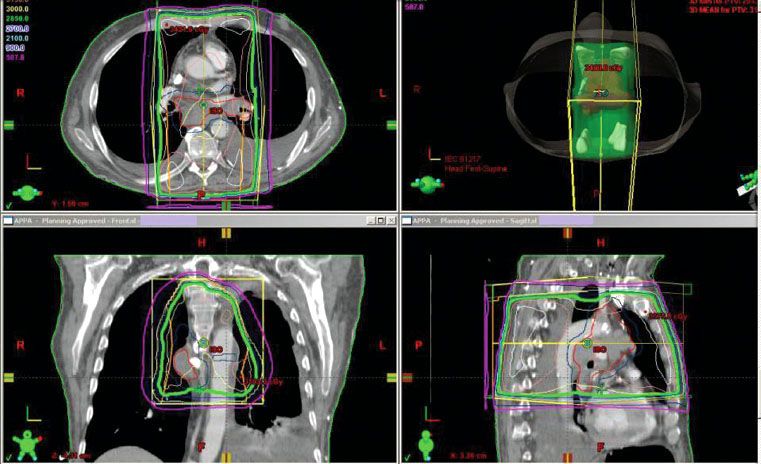
The group of patients who are referred to a radiation oncologist for palliation of liver metastases tend to be the patients who have more medical comorbidities, a greater tumor burden, and who have been previously treated with multiple other therapies. Despite these poor prognostic factors, radiation therapy can be effective in palliating symptoms (Fig. 95.2).16
Palliative treatment of the liver is limited by radiation-induced liver toxicity (RILD). This toxicity becomes apparent 1 week to 3 months after treatment and may result in liver failure and death.17 Both dose and volume of liver irradiated are important in avoiding RILD. For treatment of the whole liver, the threshold for RILD is approximately 30 Gy in 2 Gy fractions or 33 Gy in 1.5 Gy twice daily fractions.18,19 The Radiation Therapy Oncology Group’s trial RTOG-7605 evaluated multiple regimens in patients with multiple liver metastases: 30 Gy in 15 fractions, 25.6 Gy in 16 fractions, 20 Gy in 10 fractions, and 21 Gy in 7 fractions.20 Improvement in symptoms was seen in 55% of the patients, with no difference among the four treatment regimens. A subsequent RTOG trial evaluated whole-liver radiation therapy with a dose of 21 Gy in 7 fractions; patients were randomized to receive the radiation alone or with the addition of misonidazole to the radiation.21 There was minimal toxicity and a rapid symptomatic response in the majority of patients. Complete pain relief was reported in 54% of the patients, and 80% of the patients noted an improvement in pain following treatment. Overall, there was no significant benefit with the addition of misonidazole. The best response rates were seen in patients with colorectal primaries. Bydder et al.22 reported on a series of 28 patients treated with a hypofractionated regimen of 10 Gy in 2 fractions to the whole liver. The overall response rate was 54%, with two patients experiencing grade 3 toxicity (emesis and diarrhea).
Selective internal radiation therapy (SIRT) is another option for patients with diffuse liver metastases. This technique involves the use yttrium-90 (90Y) glass or resin microspheres that are given through intra-arterial infusions into the liver. A phase III trial of patients with liver metastases from colorectal cancer evaluated intra-arterial floxuridine alone compared to intra-arterial floxuridine plus SIRT with 90Y resin microspheres.23 The patients who received SIRT had a significantly better overall response rate, time-to-tumor progression, and overall survival. The addition of SIRT did not increase grade 3 or 4 toxicity.
In summary, patients with multiple symptomatic liver metastases may be palliated by whole-liver radiation therapy. Relatively low doses of 21 Gy in 7 fractions or 30 Gy in 15 fractions are generally well tolerated and provide symptomatic relief for the majority of patients.16,21
 BILIARY OBSTRUCTION
BILIARY OBSTRUCTION
Malignant biliary obstruction may be caused either by a tumor mass causing extrinsic compression or intrinsic compression from tumor progression within the bile duct lumen. This obstruction can lead to hyperbilirubinemia and jaundice, which can cause pruritus, anorexia, and weight loss. The biliary obstruction may be a life-threatening emergency. Emergent drainage of the bile duct is achieved initially by external stent. These stents are later converted to internal endoprostheses; however, these catheters typically only remain patent for 4 to 8 months.24 The patency of these stents can be extended with the use of intraluminal brachytherapy, photodynamic therapy, or external-beam radiation therapy. Intraluminal brachytherapy extends stent patency and lengthens survival duration in patients with inoperable cholangiocarcinoma.25,26 Although there are few data specifically for the use of external-beam radiation therapy for palliative treatment of metastatic biliary obstruction, it may be of benefit especially for treatment of extrinsic bile duct compression by a tumor mass.
FIGURE 95.2. Digitally reconstructed image from computed tomography simulation. The field covers the whole liver volume in a patient with multiple liver metastases from a metastatic rectal cancer.
 PAINFUL ADRENAL METASTASES
PAINFUL ADRENAL METASTASES
The adrenal glands are a common site of metastatic disease from many types of cancer, primarily due to the rich blood supply of the adrenal gland. Lung cancer is the most common source of adrenal metastases. These metastases are frequently asymptomatic, but occasionally may cause pain. For patients with a good performance status and few sites of metastatic disease, surgical management may be appropriate. Radiation therapy is also appropriate for palliative treatment.
Palliative radiotherapy typically has been given with conventional external-beam techniques. Soffen et al.27 reported the results in 16 patients with painful adrenal metastases treated with palliative radiation therapy. The majority of the patients received 30 Gy in 10 to 12 treatments. A response was seen in 12 of 16 patients (75%), with 6 of those patients having complete response for the duration of their survival. Nearly half of the patients (44%) experienced nausea, which may have been related to the technique of treatment with anterior and posterior fields. No late toxicity was seen, but the median survival was only 3 months. There are multiple recent reports evaluating the use of intensity-modulated radiation therapy techniques or stereotactic body radiation therapy techniques for treatment of adrenal metastases. Most of these patients were treated for asymptomatic adrenal metastases, rather than for palliation of symptoms. These treatments appear to provide a high rate of local control, and may be appropriate for patients with limited volume disease (oligometastases). In the palliative setting, there is a benefit with a dose of 30 Gy in 10 to 12 treatments using conventional techniques.
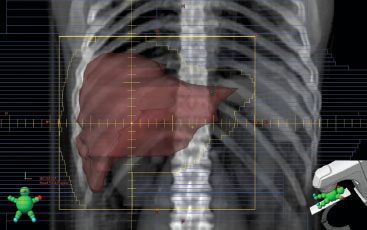
FIGURE 95.3. Digitally reconstructed rendering of an anterior-posterior treatment field of the spleen. Splenomegaly may respond rapidly to low doses of radiation therapy. The fields should be checked clinically on a frequent basis to determine if the blocks or multileaf collimators need to be adjusted.