Pain Management
INTRODUCTION
Cancer causes pain. Although studies give varying results, even the most conservative studies report that at least 20% of patients have pain at diagnosis or in the advanced stages of their illness. Numerous studies show that clinicians often undertreat cancer pain and that undertreated pain causes undue burdens on patients and their families. Clinicians may underestimate how much pain patients feel, or not be facile in pain management techniques that have the potential to greatly improve a patient’s quality of life. Patients’ misconceptions about pain medications also contribute to inadequate pain management. Patients may be reluctant to take pain medications for fear of addiction or worry that requiring pain medication indicates that death is imminent. Patients at particular risk for undertreatment include women, minorities, the poor, and the old (1).
PAIN ASSESSMENT
With simple treatments, more than 80% of patients can have their pain controlled (2). The first step in devising a pain management plan requires the patient to characterize the pain. It is helpful to distinguish neuropathic pain, which patients will characterize as burning, sharp, or shooting, from visceral and somatic pains, which are dull and aching. In a cancer patient, pain is usually caused by chemotherapy, radiation therapy, or tumor recurrence (3). Understanding the origin of the pain aids in the development of the appropriate pain management plan. Pain from bony metastases, for example, may require an NSAID or radiation, whereas pain from a local recurrence outside of the bone may require only opioids.
The next step is to assess the baseline level of pain using simple, validated methods such as visual analog scales, numerical scales (e.g., from 1 to 10), or pictoral scales (faces, circles of different colors). Many clinicians are unaware that patients with chronic pain will often lack physiologic signs that may indicate pain. Patients with chronic pain rarely show signs of sympathetic arousal such as tachycardia or hypertension. Patient self-report is the gold standard of pain assessment.
A patient’s experience of pain is not limited to physical sensation. Depression, anxiety, and existential distress can all exacerbate a patient’s perception of and ability to cope with pain. Because psychosocial and spiritual factors play such a large role in a clinician’s ability to treat pain, it is critical to include assessment of these factors when developing a comprehensive pain management plan. The comprehensive clinical assessment should therefore include the following:
• Psychological, social, financial, and spiritual sources of coping and of distress
• An assessment of mood disorders: screen for and treat depression and anxiety
• Evaluating how the patient and family are coping with the illness
TREATMENT
The goal of treatment is to adequately manage the pain while trying to minimize toxicity. For moderate pain or pain that persists despite NSAIDs or acetaminophen, it is appropriate to initiate short-acting opioids in conjunction with NSAIDs or acetaminophen (3, 4). Short-acting opioids such as morphine, oxycodone, or hydromorphone have an onset of action of 15–30 min, reach peak effect in about 60 min, and have a duration of action of approximately 4 h. Consider the patient’s preferences and experience when choosing an opioid, and avoid codeine except in a patient who has used it with success. Short-acting opioids are dosed every 3–4 h and delivered via the least invasive route that works, preferably oral.
 STARTING LONG-ACTING OPIOIDS
STARTING LONG-ACTING OPIOIDS
For a patient with intermittent pain, short-acting opioids may suffice. However, patients with continuous pain or with a combination of continuous and intermittent worsening pain will need to begin long-acting opioids. Long-acting opioids such as sustained-release oxycodone (Oxycontin) or sustained-release morphine (MS Contin) have an onset of action of about 1 h, reach peak effect in 3–4 h, and have a duration of action of 8–12 h.
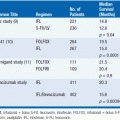
Choosing the appropriate long-acting opioid dose requires multiple steps. First, determine the total daily dose of short-acting opioid required to provide adequate analgesia. Second, choose the long-acting preparation. Finally, convert the short-acting opioid into the appropriate dose of the long-acting preparation. Commonly morphine or oxycodone is used in a short-acting preparation, and both have long-acting preparations that are inexpensive and well tolerated. When using morphine or oxycodone, the total daily dose of short-acting opioid can be directly converted to long-acting opioid. For example, a patient requiring 5 mg of oxycodone every 3 h is taking a total of 40 mg of oxycodone in a 24-h period. This patient may be started on 20 mg of long-acting oxycodone every 12 h. Alternatively, if the patient requires a different long-acting opioid, it is possible to determine the correct dose using equianalgesic tables (Table 21-1). When converting a patient from one opioid to another, it is important to account for incomplete cross-tolerance by decreasing the dose of the new opioid by 25%–50%.
 CASE EXAMPLE
CASE EXAMPLE
A 45-year-old woman with breast cancer metastatic to her ribs presents to clinic and describes aching pleuritic chest pain that is worse with movement. For pain she takes 4 mg of oral hydromorphone (Dilaudid) every 6 h around the clock. She complains that her pain medication wears off, and she is frequently in pain. What are the problems with her pain management? How do you convert her to a long-acting opioid and what breakthrough dose do you give?
Her first problem is that she is not taking an adjuvant that would minimize the toxicity of the opioid. Start an adjuvant medication such as acetaminophen or an NSAID. An NSAID would be a good choice in this patient because NSAIDs are particularly effective for bone metastases. Overall, no NSAID works better than any other. However, individual patients may do best with a particular NSAID. If this works, she could take less opioid.
Her second problem is that with her persistent pain, her current dosing interval of 6 h is too long: the short-acting opioid has worn off in 4 h. She could take it more frequently, but every 4-h dosing is cumbersome and interrupts sleep. She needs a long-acting opioid. Since hydromorphone has no long-acting formulation, switch her medication to sustained-release oxycodone (Oxycontin), sustained-release morphine (MS Contin), or transdermal fentanyl. Of these choices, transdermal fentanyl is more difficult to titrate in a patient who is requiring multiple changes in dosing. Oxycodone and morphine, in their respective doses, are similarly effective and easy to titrate. Here is the procedure for changing her medication from oral hydromorphone to long-acting oral morphine:
1. Calculate her 24-h short-acting opioid dose:
4 mg hydromorphone × 4 doses/24 h = 16 mg/24 h
2. Look up the equianalgesic conversion from oral hydromorphone to oral morphine:
hydromorphone 7.5 mg oral = morphine 30 mg oral
3. Set up an equation to convert the dose:
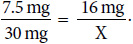
Solve for X:
X = 64 mg morphine in 24 h
4. Reduce this dose by 25%–50% due to incomplete cross-tolerance between the old and new opioids. Choosing the lower reduction of only 25%, the final dose is computed as follows:
0.25 × 64 mg = 16 mg reduction
64 mg – 16 mg = 48 mg oral morphine in 24 h
5. Divide the 24 h total into divided doses. Long-acting morphine can be given q8 or q12. Note: these medications should not be dosed tid, but rather q8h to ensure adequate analgesia and to decrease sedation. For this example, the q8h dosing is 48 mg/3 = roughly 15 mg long-acting morphine every 8 h.
 BREAKTHROUGH DOSING
BREAKTHROUGH DOSING
All long-acting opioids must be paired with an appropriate dose of short-acting medication in the event that the patient experiences breakthrough pain. The usual dose for breakthrough pain is 10%–20% of the total 24-h dose. Opioids prescribed for breakthrough pain may be safely dosed as often as every 1–2 h. Short-acting opioids that are orally dosed reach peak analgesic effect at 1 h and last no more than 4 h. In this example, the patient is taking 45 mg of oral long-acting morphine per day. An appropriate dose for breakthrough pain would be 10%–20% of the total daily long-acting dose or 5–10 mg every 3–4 h as needed.
Taking excessive doses of breakthrough medication can be a symptom of poorly controlled pain. Ideally a patient would not need more than 2–3 breakthrough doses over 24 h. If a patient requires more frequent dosing, the long-acting dose may be too low. To calculate the increase in the long-acting dose, add up the total amount of breakthrough medication taken in 24 h and convert it to the appropriate amount of the long-acting formulation that the patient is already taking. In this example, if your patient consistently took 5 mg of morphine every 4 h for breakthrough pain, the 24-h total (30 mg) should be given instead as long-acting morphine. Her previous dose of morphine 15 mg q8h could be increased to 30 mg in the morning and evening and 15 mg in the afternoon.
SIDE EFFECTS
Common side effects of opioids include nausea, vomiting, sedation, and constipation. Nausea and sedation usually resolve within 1 week. In most cases no psychoimpairment remains 2 weeks after the change in the opioid dose. Constipation, unlike other side effects, does not attenuate with long-term use. Therefore, all patients on opioids require a bowel regimen that includes a stimulant laxative (e.g., senna 2 tab po bid) and stool softener (e.g., docusate [Colace] 100 mg po bid).
 SAFETY
SAFETY
Care must be taken when dosing opioids in elderly patients or in those with hepatic or renal insufficiency as they will be more sensitive to the medication and experience side effects such as sedation at lower doses. Start at a low dose and increase slowly. The key to safe and competent pain management is assessment and frequent reassessment. Also, watch for delirium and sedation in patients taking concomitant benzodiazepines. Delirium can sometimes improve with a slight reduction in the opioid.
 OVERSEDATION
OVERSEDATION
Opioid respiratory depression is due to generalized CNS depression. Therefore, when respiratory depression is due to an overdose of opioids, it is always preceded by somnolence. Respiratory depression due to opioids does not commonly occur in patients on a stable dose of opioids unless the patient is experiencing alterations in metabolism or excretion of the drug, has been given another sedating medication such as benzodiazepines, or has developed a new medical complication such as infection. Therefore, if sedation does occur, it is important to do a full medical evaluation. In the sedated but clinically stable patient, initial management includes discontinuation of the opioid, supplemental oxygen, and efforts to arouse the patient. For significant respiratory depression, dilute 0.4 mg naloxone into 10 cc of normal saline and administer 1 cc every 1–2 min until the respiratory rate is satisfactory. The clinical goal is to reverse the respiratory depression without reversing the analgesic effect of the opioid. Beware that too rapid infusion of naloxone may precipitate a pain crisis in a patient on chronic opioids.
DIFFICULT-TO-CONTROL PAIN
Even with standard pain control, some patients still suffer. Patients with severe pain may need rapid titration of opioid medications; for protocols, see the NCCN guidelines on adult cancer pain (www.nccn.org). When pain is difficult to control, consider the following etiologies: neuropathic pain, incident pain, tolerance, addiction, somatization, and (the most frequent) undermedication.
• Neuropathic pain. Treat with effective adjuvants such as gabapentin (start 300 mg PO qhs and increase gradually to 300–600 mg po tid, max 3600 mg/day) or tricyclic antidepressants (nortriptyline 10 mg PO qhs and increase to therapeutic dose). Methadone, which blocks opioid and NMDA receptors, may also be helpful.
• Incident pain (such as before dressing changes or planned activity). Provide quick-onset, short-acting analgesia with an extra dose of short-acting opioid (use the breakthrough dose, 10%–20% of the daily opioid dose). For incident pain due to bony metastases, consider adjuvants such as NSAIDs (ibuprofen 600–800 mg po tid) or radiation treatment.
• Tolerance. Consider increasing the opioid dose or changing the opioid.
• Psychological addiction. It is rare in cancer patients treated with opioids (6), but patients who have a history of substance abuse have a higher risk (7).
• Somatization. Consult the psychiatry, pain, or palliative-care services.
FENTANYL PREPARATIONS
Transdermal fentanyl, a long-acting fentanyl preparation available in a patch, is changed every 48–72 h (7). It is ideal for patients who have trouble taking oral medication, due to nausea, difficulty swallowing, or gastrointestinal tract dysfunction. Fentanyl, a lipophile, diffuses into the fat of the skin and then into the bloodstream, so place the patch over an area of the body with subcutaneous fat such as the abdomen, upper arm, or buttocks. The patch is not an effective initial treatment for severe pain because at least 12 h are required to build enough drug in the fat reservoir to establish adequate blood levels. Frequent rescue dosing is needed for the first 12–24 h until the fentanyl accumulates. Because the patches need a reservoir of subcutaneous fat, they are not recommended for patients who weigh less than 110 lb or for older cachectic patients. In contrast, febrile patients may have increased absorption and suffer possible toxicity.
Transmucosal immediate release fentanyl (TIRF) products such as Actiq and Fentora have an FDA black box warning and should be used with caution. Because life-threatening hypoventilation could occur at any dose in patients not taking chronic opioids, TIRF is indicated for breakthrough cancer pain only in patients who are already receiving and who are tolerant to opioid therapy. Patients considered opioid tolerant are those who are taking at least 60 mg morphine/day, or an equianalgesic dose of another opioid, for a week or longer. In addition, TIRF products are not interchangeable and converting from one TIRF product to another must be done according to labeled dosing instructions.
METHADONE
Rotation to methadone is complex because methadone has a long half-life and accumulates in the fat stores, and its potency increases when a patient is taking another opioid. Methadone can be up to 10 times more powerful in patients on high doses of opioids than in patients who are opioid naive or on lower doses (Table 21-2). Contact a pain or palliative-care specialist for assistance when converting to methadone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree