OVARIAN STEROIDOGENESIS
LIPOPROTEINS AS CHOLESTEROL SOURCE
Lipoproteins are complex particles containing a lipid core surrounded by amphiphilic proteins and phospholipids (see Chap. 162). Low-density lipoprotein (LDL; density = 1.019–1.063 g/mL) is the predominant cholesterol and cholesteryl ester carrier in human plasma and provides cholesterol to cells for membrane synthesis and as substrate for steroidogenesis in steroidogenic organs such as the ovary.78 High-density lipoprotein (HDL; density = 1.063–1.21 g/mL) also carries cholesterol and cholesteryl ester. In humans, HDL cannot provide cholesterol for steroidogenesis.79,80 In rats, a model often used in the study of the ovary, HDL and LDL can provide cholesterol for steroid hormone production.81
All cells can obtain cholesterol from two sources. Cholesterol can be synthesized de novo from acetate by the cell, or the cell can obtain the cholesterol from an external source such as lipoprotein.79 The mechanism by which steroidogenic cells in the human ovary obtain LDL-cholesterol essentially is the pathway described by Brown and Goldstein82 in their studies of the human fibroblast. LDL binds to ovarian steroidogenic cell-membrane receptors with high affinity and specificity. These receptors recognize apolipoprotein B, the predominant LDL apoprotein.78 In studies of human ovarian corpora lutea membranes, the number of LDL receptors is highest in the midluteal phase, suggesting that LDL-receptor number and the rate of steroidogenesis are positively correlated.83
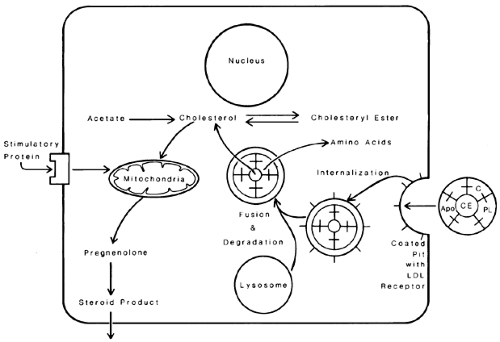
After the LDL particle binds to the receptor on the plasma membrane, particle and receptor are internalized by the cell inside coated vesicles, which fuse to form lysosomes. The lysosomes hydrolyze the components of the LDL particle; cholesteryl ester is hydrolyzed to free cholesterol, and apoproteins are hydrolyzed to amino acids. The free cholesterol can be stored in the cell as cholesteryl ester or can be converted to steroid hormone products. The increase in the cell concentration of free cholesterol or a metabolic product such as an oxygenated sterol acts as a major control point in cell cholesterol metabolism by decreasing the number of LDL receptors, decreasing de novo cholesterol synthesis from acetate and increasing esterification of cholesterol to cholesteryl ester.84,85 In this way, the cell prevents the overaccumulation of cholesterol (Fig. 94-20).
The factor that down-regulates de novo synthesis of cholesterol and LDL receptors appears to be an oxysterol rather than
cholesterol itself. A prime candidate for this regulatory role is 26-hydroxycholesterol. Luteinized human granulosa cells contain 26-hydroxylase messenger RNA (mRNA), and this enzyme is localized to mitochondria. Data suggest that, when steroidogenesis is active, the 26-hydroxylase enzyme is inhibited by the products of the side-chain cleavage enzyme, allowing de novo cholesterol synthesis and increased LDL-cholesterol uptake.86 When steroidogenic activity is decreased in the ovary (i.e., follicle or corpus luteum), 26-hydroxylase activity remains, allowing the formation of 26-hydroxycholesterol and the resultant reduction in cholesterol synthesis and LDL-receptor gene expression. This finely tuned series of regulatory steps ensures enough cholesterol for cell function (e.g., membrane synthesis and specialized activities, such as steroid hormone production), but it prevents the overaccumulation of cholesterol in the cell.
cholesterol itself. A prime candidate for this regulatory role is 26-hydroxycholesterol. Luteinized human granulosa cells contain 26-hydroxylase messenger RNA (mRNA), and this enzyme is localized to mitochondria. Data suggest that, when steroidogenesis is active, the 26-hydroxylase enzyme is inhibited by the products of the side-chain cleavage enzyme, allowing de novo cholesterol synthesis and increased LDL-cholesterol uptake.86 When steroidogenic activity is decreased in the ovary (i.e., follicle or corpus luteum), 26-hydroxylase activity remains, allowing the formation of 26-hydroxycholesterol and the resultant reduction in cholesterol synthesis and LDL-receptor gene expression. This finely tuned series of regulatory steps ensures enough cholesterol for cell function (e.g., membrane synthesis and specialized activities, such as steroid hormone production), but it prevents the overaccumulation of cholesterol in the cell.
Although there are receptors for HDL in the human ovary, HDL is unable to provide cholesterol for steroid hormone production. At high concentrations, HDL seems to inhibit steroidogenesis by cultured human ovarian cells.80 This differential effect of HDL and LDL on steroid hormone synthesis by human ovarian cells could be of physiologic importance because of compartmentalization within the ovary. Granulosa cells in the preovulatory graafian follicle are bathed in follicular fluid but are separated from the ovarian vasculature by the basal lamina of the follicle. Human follicular fluid contains little or no LDL, but contains levels of HDL close to those in the plasma. The lack of available LDL probably limits progesterone synthesis before ovulation. After ovulation, the follicle becomes the vas-cularized corpus luteum, exposing these granulosa cells to plasma levels of LDL. The rapid rise in ovarian progesterone production after ovulation can be explained, at least in part, by the sudden availability of LDL-cholesterol as substrate by means of the LDL receptor.87 Before ovulation, the theca is well vascularized, and LDL would be available to provide cholesterol for thecal androgen production. Androgen can cross through the basal lamina to provide substrate for estrogen production by follicle granulosa cells. The theca of the dominant follicle has the richest blood supply of all follicles, ensuring adequate substrate for estrogen synthesis.88
Rat ovarian steroidogenic cells also can use LDL-cholesterol by the pathway described for human cells. However, HDL also can provide cholesterol for rat ovarian steroidogenesis by a mechanism that is quite different from that described for LDL. For example, the HDL particle can provide cholesterol to the cell in the absence of degradation of the apolipoprotein surface coat.81 The HDL (scavenger, type 1)-receptor mRNA is localized to theca cells of the rat ovary. Gonadotropic stimulation with hCG causes a marked increase in HDL receptor mRNA in theca interstitial cells and luteinized granulosa cells, consistent with a functional role for this receptor in rodent cholesterol transport and ovarian steroidogenesis.89
MECHANISMS OF GONADOTROPIN EFFECTS
The α and the β subunits of FSH and LH are required for binding to the specific membrane receptors. Theca cells have specific LH receptors, but granulosa cells contain FSH receptors. FSH stimulates production of granulosa cell LH receptors. Gonadotropin binding to its receptor stimulates the cAMP–A kinase regulatory system.90 By this mechanism, gonadotropins increase available free cholesterol by increasing lipoprotein receptor number and by increasing hydrolysis of stored cholesteryl ester. In the absence of available lipoprotein (e.g., under serum-free in vitro conditions, perhaps within the follicle in the human ovary before ovulation), gonadotropins stimulate cholesterol synthesis de novo from acetate in the cell. The free cholesterol is transferred to mitochondria, which is the location of the rate-limiting enzyme in steroidogenesis, P450 side-chain cleavage enzyme (P450scc), which converts cholesterol to pregnenolone.81 There is evidence that cholesterol is carried to the mitochondria by a carrier protein called sterol carrier protein-2 (SCP-2). Gonadotropins then facilitate the transport of cholesterol from the outer to the inner mitochondrial membrane and continued transport to the P450scc enzyme on the inner mitochondrial membrane.91 A protein named steroidogenic acute regulatory protein (StAR) has been implicated as the regulator of cholesterol translocation from the outer to inner mitochondrial membrane.92 StAR mRNA transcripts are localized in the human ovary to the theca of preovulatory follicles, and luteinized granulosa and theca cells in the corpus luteum.93 Mutations of the StAR gene result in congenital lipoid adrenal hyperplasia, in which synthesis of all gonadal and adrenal steroids is severely impaired.94 This finding establishes the critical role of StAR protein in steroidogenesis. StAR gene expression is stimulated by cAMP.93 Gonadotropins can stimulate acutely the transport of cholesterol to the P450scc enzyme and chronically increase the amount and activity of this enzyme. After conversion of cholesterol to pregnenolone in the mitochondria, the pregnenolone moves back out of the mitochondria and into the cell cytoplasm for conversion to progesterone in corpus luteum cells and androgen in theca cells. The enzymes for these conversions are located on the microsomes. A summary of cholesterol transport and metabolism within ovarian cells is shown in Figure 94-21.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree