Chapter 59 Ovarian Cancer
Ovarian cancer is the fifth most common cause of cancer death among women, and the leading cause of gynecologic cancer-related death in the United States.1 The incidence of ovarian cancer for 2010 in the United States was projected to be 21,880 cases, thus constituting 3% of all cancer diagnosed in women. The lifetime risk in the general population is 1.4%. In 2010 the expected number of deaths attributed to ovarian cancer was projected to be 13,850, accounting for 5% of all cancer-related deaths.2
Epidemiology and Etiology
Epidemiology
Ovarian cancer is infrequent before the age of 40, with a median age at diagnosis of 63 years. The age-specific incidence increases with age, reaching its peak in the eighth decade, with an incidence of 57 per 100,000 in the 70- to 74-year age-group3 (Fig. 59-1). Incidence rates and the number of cancer-related deaths have been relatively stable over the past three decades.
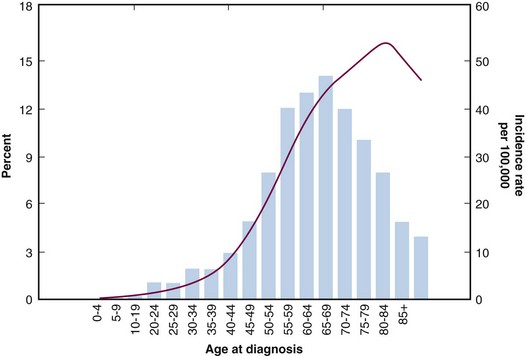
Figure 59-1 Incidence rates by age for ovarian cancer.
Data from Yancik R, Ries LG, Yates JW: Ovarian cancer in the elderly. An analysis of Surveillance, Epidemiology, and End Results Program data. Am J Obstet Gynecol 154:639-647, 1986.
Five-year overall survival (OS) is typically reported for epithelial ovarian cancer and is subcategorized by stage; for all stages it is approximately 45%. The survival is significantly better for localized disease, with a 5-year OS of 93%. However, approximately 70% of women are diagnosed when the disease has spread, with 5-year OS of 20% to 30%.1,4–6 Women diagnosed when younger than age 65 have higher 5-year OS (65.8%) in comparison to women older than age 65 when diagnosed (32.9%).
Etiology
Environmental Risk Factors
Country of residence and race has been associated with an increased risk of ovarian cancer. Women living in North America and in northern Europe have the highest risk. The incidence is highest among white women and is intermediate among black women, whereas Hispanic, Asian, and Native American women have the lowest reported risk of ovarian cancer.7 Many dietary factors have been evaluated in regard to the risk of ovarian cancer, including a diet high in meat and calories, antioxidant intake (e.g., vitamins A, C, and E), and lactose or coffee consumption, although none has consistently been shown to significantly alter the risk.7–10
Hormonal Factors
Hormonal factors including increased parity, age at first birth before age 25 years, history of breast feeding, oral contraceptive use, and tubal ligation have been shown in multiple epidemiologic studies to decrease the risk of developing ovarian cancer by 25% to 60%.11–13 Hormone replacement therapy has been shown in the Million Women Study and in a more recent Danish cohort study to be a risk factor for ovarian cancer.14,15 Both studies demonstrated a 1.5- to 2-fold increased risk of developing ovarian cancer in current users of hormone replacement therapy. The protective effects of parity, multiple births, oral contraceptive use, and breast feeding support the “incessant ovulation” theory, in which each ovulatory cycle causes damage to the ovarian epithelium and then the process of aberrant repair becomes an initial step in carcinogenesis.16 Therefore, the more ovulatory cycles a woman has in her lifetime the higher her risk of developing ovarian cancer. A second hypothesis attributes an increased risk of ovarian cancer to excessive gonadotropin secretion.17 Because of the high concentration of gonadotropins, estrogenic stimulation occurs, leading to the entrapment of epithelial cells in inclusion cysts, which further leads to cell proliferation and malignant transformation.17,18 Oral contraceptive use and pregnancy, in addition to stopping ovulation, lower gonadotropin levels; therefore, both traditional risk reducers are in concordance with both theories. Hence, patients treated for infertility with ovarian stimulation would be expected to have an increased risk of ovarian cancer. An increased risk of ovarian cancer, although predominantly borderline tumors, was reported in a cohort of women treated for infertility with prolonged use of clomiphene citrate, an ovulation stimulator, in comparison to the general population.19 However, the data have not been consistent: a recent publication of a large Danish population-based cohort study did not support the correlation between infertility medication and ovarian cancer.20
Genetic Factors
Fifteen percent of all ovarian cancers are attributable to known genetic mutations, that is, 13% to the hereditary breast/ovarian cancer syndrome (BRCA1 and BRCA2 germline mutations) and 2% to the hereditary nonpolyposis colon cancer syndrome (MLH1, MSH2, MSH6 mutations).19–21 Women with hereditary nonpolyposis colon cancer syndrome (HNPCC) have an increased risk of developing multiple types of cancer, with colon, endometrial, and ovarian cancer being the most common. The lifetime risk of women with HNPCC of developing ovarian cancer is 15% to 25%.22 The frequency of BRCA mutations differs among ethnic groups. Founder (recurrent) mutations have been identified in specific ethnic groups; for example, up to 40% of all Ashkenazi Jewish women with ovarian cancer carry one of three founder mutations.23 Additional founder mutations have been found among Polish and French-Canadian women.24,25 Women who harbor a BRCA1 mutation have a lifetime risk of 30% to 40% for developing ovarian cancer. Women with a BRCA2 mutation have a 15% to 25% lifetime risk of developing ovarian cancer, depending on the location of the mutation in the gene; that is, a higher risk is correlated with a mutation in the ovarian cancer cluster region.26 Women with a BRCA1 or BRCA2 mutation also have an increased risk of fallopian tube cancer.29 The lifetime risk of breast cancer for carriers of BRCA1 and BRCA2 mutations is 50% to 85%.
Prevention and Early Detection
Screening and Prophylactic Surgery
The majority of women with ovarian cancer present with advanced disease and therefore a poor long-term prognosis. In contrast, those patients who are diagnosed when the cancer is limited to the ovaries have a good prognosis, with a 5-year OS of 80% to 90%.27 Hence, the focus of ovarian cancer screening is to identify women with early-stage disease. The most common techniques used to date in screening are serum CA 125 and ultrasonography. The CA 125 serum tumor marker is an antigenic determinant detected by radioimmunoassay that is elevated in 80% of epithelial ovarian cancers.
Two large randomized trials are now being performed to address the question of the yield of screening for ovarian cancer: the U.S. National Institutes of Health (NIH) Prostate Lung Colorectal and Ovary Study (PLCO) and the U.K. Collaborative Trial of Ovarian Cancer Screening (UKCTOCS).28,29 UKCTOCS randomly assigned 202,638 postmenopausal women to a control group (no screening) versus two different arms of screening (annual transvaginal ultrasonography or annual CA 125 screening and further transvaginal ultrasonography if results were elevated). PLCO randomly assigned 39,115 women to screening (annual CA 125 and transvaginal ultrasonography) versus no screening. The trials to date have only reported prevalence data showing, in both trials, that the majority of the cases diagnosed were at advanced stage. The effect of screening on ovarian cancer mortality is pending long-term follow-up.
Multiple trials have shown a low positive predictive value (PPV) of screening the general population and an expected higher PPV when screening high-risk populations.30–35 Until further evidence is available, screening in the general population is not recommended. For patients with an identified BRCA mutation, screening has been recommended at the NIH Consensus Conference on Ovarian Cancer despite the absence of proven benefit.36
While large-scale screening trials using CA 125 and ultrasonography are underway, a new direction examining potential novel serum markers using proteomics and genomics is being explored.37–39 The majority of studies are evaluating the sensitivity and specificity of combinations of markers. Visintin and associates,38 from Yale University, studied concentrations of a combination of six proteins, including leptin, prolactin, osteopontin, insulin-like growth factor 2, macrophage inhibitory factor, and CA 125, in 156 newly diagnosed ovarian cancer patients and 362 healthy controls. They reported a sensitivity of 95.3% and a specificity of 99.4% for the detection of ovarian cancer. Although these results are promising, additional validation studies are needed.
The value of referring patients with an identified BRCA mutation to genetic counseling has been established, because the decision making is complex. Individualized management recommendations are made after evaluating the patient’s specific risk, her age, and her reproductive plans. The efficacy of prophylactic salpingo-oophorectomy has been documented with a reduction in gynecologic cancer by 85% to 96%.40,41,42,43 The risk of ovarian and fallopian tube cancers is profoundly diminished, but there remains an approximately 1% risk of primary peritoneal cancer.40,41,42 In addition, the risk of developing subsequent breast cancer was reduced by 50% to 60%.44 The NIH consensus panel in 1995 recommended prophylactic oophorectomy for high-risk women at age 35 or after the completion of childbearing.36
Biologic Characteristics and Prognostic Factors
Clinicopathologic Factors
The staging of ovarian cancer is surgical and is directly correlated with survival. The 5-year OS of patients diagnosed between 1995 and 2006, published in the Surveillance, Epidemiology and End Results (SEER) database, was 45.8% for all stages; 93.8% for stage I, 72.8% for stage II, and 28.2% for combined stages III and IV.45
Histologic grade, based in the FIGO system on architectural features, that is, the ratio of glandular or papillary structures versus solid tumor growth within a tumor, is an important prognostic factor in epithelial ovarian cancer.46–48 Patients with high-grade (poorly differentiated) tumors have worse outcomes than those with low-grade (well-differentiated) tumors. In patients with early-stage (IA to IB) low-grade tumors a 94% 5-year OS has been reported with surgery alone.49
The effect of histologic cell type has generally been of less important prognostic significance than other factors. However, it has been suggested that early-stage endometrioid and mucinous cell types have a better prognosis than serous and clear cell adenocarcinoma; but when grade and cell type are evaluated, histologic cell type has not been found to be an independent factor.50,51
In patients with advanced-stage disease, chemotherapy sensitivity is an important prognostic factor. Therefore patients with serous histology have a better prognosis than those with the more chemoresistant tumors (e.g., mucinous and clear cell).52
Age at diagnosis and performance status have been shown to be independent prognostic factors in several reports.53,54 The prognostic significance of tumor size and bilaterality has not been proven.
The volume of residual tumor at the completion of a cytoreductive surgery for advanced ovarian cancer is directly correlated with survival.53 Chi and colleagues55 from Memorial Sloan-Kettering Cancer Center have shown that an aggressive primary cytoreduction, including extensive upper abdominal procedures, resulted in increased optimal cytoreduction rates and significantly improved progression-free survival (PFS) and OS in patients with advanced ovarian carcinomas. However, it remains controversial if it is primarily the aggressiveness of the surgery or the biology of the tumor or a combination of the two that make this contribution to prognosis.
Preoperative and postoperative CA 125 levels, a reflection of volume of disease, and the time frame over which the level declines during chemotherapy have been shown to correlate with prognosis.56
Biologic Factors
The prognostic significance of cellular DNA content of epithelial ovarian cancer as determined by flow-cytometric analysis has shown that aneuploid (vs. diploid) DNA content in ovarian tumors is correlated with more aggressive biologic behavior and, therefore, a worse clinical course.57,58 Multivariate analysis has found diploidy to be an independent prognostic factor.58
Molecular markers, including both abnormalities in oncogene products (e.g., ERBB2 [HER2/neu], ERBB3 [HER3]) and suppressor gene products (e.g., TP53), have been shown to have an impact on the prognosis of epithelial ovarian cancer.59,60 Many additional biologic markers including markers of proliferation and of DNA repair, together with newer studies on loss of human leukocyte antigen class I, KLK6, and KLK13, have been reported,61,62 although their significance has not yet been established.
Pathology and Pathways of Spread
Pathogenesis
Ovarian cancers are classified by their histology. The largest categories are epithelial, germ cell, and sex cord/stromal. Epithelial ovarian cancers account for approximately 80% of all ovarian cancers. The traditional view of the pathogenesis of epithelial ovarian cancer asserts that all tumor subtypes share a common origin in the surface epithelium of the ovary. Ovulation inflicts trauma on the surface epithelium and releases inflammatory cytokines, capable of damaging DNA, that lead to a process of repeated damage and repair.63,64 This increases the susceptibility of ovarian epithelial cells to malignant transformation. Therefore, incessant ovulation increases the risk of developing epithelial ovarian cancer. Moreover, numerous invaginations into the stroma develop as the ovary ages. These invaginations further form cortical inclusion cysts in the stroma.65 The epithelial cells in the cysts are then exposed to high levels of hormones that induce proliferation. If the cells have previous DNA damage, they are then susceptible to malignant transformation.
Although traditionally it is thought that serous ovarian cancers arise either in an inclusion cyst within the ovary or in the surface epithelium cells, there is little evidence to support the existence of an identifiable precursor lesion on the ovary. As an increasing number of women have undergone prophylactic surgery for genetic predisposition to breast and ovarian cancer, pathologists have had the opportunity to evaluate the removed ovaries and fallopian tubes for the presence of early cancers or noninvasive lesions. In several studies, isolated foci of cancer have been seen in the fallopian tube but rarely have small isolated cancers been found confined to the ovary.66–68 These observations led many authors to conclude that, in many cases, the fallopian tube is the primary site of cancer, which if diagnosed later in the course of disease would have been classified as a primary ovarian cancer.68–70
Pathways of Spread
Histologic Classification
Epithelial ovarian carcinomas are subclassified based on histologic cell type: serous, mucinous, endometrioid, clear cell, and transitional. They are also divided into borderline (also called tumors of low malignant potential) and invasive. Borderline tumors of the ovary are defined as those with epithelial atypia without stromal invasion. They generally are associated with an excellent prognosis, with a nearly 100% 5-year OS when diagnosed at stage I.71 Borderline tumors occur primarily in women of a younger age in comparison with invasive cancer.
Serous carcinoma is the most common histologic subtype of epithelial ovarian cancer.72 It is characterized histologically by broad, branching papillae covered with stratified epithelial cells. High-grade serous carcinoma contains large pleomorphic nuclei with many mitoses. Papillary fenestrations with slit-like fenestrations are common, there may be large areas of necrosis, and psammoma bodies may be present.
Low-grade serous ovarian carcinoma (micropapillary carcinomas) are tumors with grade 1 nuclear atypia, low-grade architecture, and low mitotic activity.73 They are associated with extensive papillary architecture and often contain an abundance of psammoma bodies. Low-grade serous carcinomas may arise in association with borderline tumors and are distinct from high-grade serous tumors, because they do not behave aggressively. The low-grade and borderline serous tumors frequently have mutations in BRAF and KRAS and lack TP53 mutations, as opposed to the high-grade serous tumors. Borderline serous ovarian tumors clinically behave in an intermediate manner between benign and malignant; that is, there may be peritoneal implants but for the majority the implants are noninvasive. The 5-year OS of stage III borderline serous tumors is approximately 85%, whereas that of stage III invasive serous cancer is a mere 20% to 30%.6,74 Long-term follow-up of 276 patients with ovarian serous tumors of low malignant potential identified unresectable disease and invasive implants as significantly associated with decreased survival (p <.001).78
Mucinous cystadenocarcinomas comprise approximately 15% of ovarian tumors. The majority are benign (80%), approximately 10% are borderline, and 10% are invasive. Mucinous tumors are diagnosed when the majority of the epithelial cells contain mucin. On gross examination they are the largest of all ovarian tumors, are commonly unilateral, have a smooth surface, and are multilocular. Microscopically, the malignant areas show back-to-back glands with malignant cells and little or no stroma. The epithelial cells are columnar or polygonal, with eosinophilic or mucinous cytoplasm. Goblet cells and signet ring cells may be present, although if they are abundant a metastasis from a gastrointestinal origin should be considered. Invasive mucinous carcinoma of ovarian origin is rare and is usually diagnosed at an early stage.75 Molecular studies have shown a link between borderline and invasive mucinous tumors.76 Unlike serous ovarian cancers, individual tumors frequently show heterogeneity with benign, borderline, and invasive areas.
Endometrioid carcinomas comprise approximately 10% of all epithelial ovarian cancers and commonly arise in extrauterine endometrial tissue. Approximately one third of the cases are associated with endometriosis.77 Adenocarcinomas associated with endometriosis may develop in association with proliferative adenofibromas and frequently occur after de novo malignant transformation of the endometrial epithelium. They are composed of epithelial cells and patterns similar to endometrial hyperplasia and neoplastic changes of the endometrium. Early-stage endometrioid carcinomas are bilateral in approximately 15% of cases. They are both cystic and solid and often are seen near foci of endometriosis. The grading is identical to that of primary carcinomas of the endometrium. Endometrioid tumors of the ovary carry genetic alterations in the phosphatase and tensin homolog (PTEN) similar to those in the endometrium.
Clear cell carcinomas of the ovary are adenocarcinomas in which most of their epithelial cells have clear cytoplasm, predominantly owing to glycogen, although part may be from mucin. They are easily recognizable under low power, owing to their unique architecture. They primarily have a papillary pattern with hyalinized stroma, although they can also have a more cystic, glandular, or solid pattern. Clear cell carcinomas are uncommon, comprising approximately 5% of all epithelial ovarian tumors in North American, and almost half are associated with endometriosis. Patients predominantly present with stage I disease (50% to 60%); and when compared stage for stage to serous ovarian cancer, the prognosis is worse.78,79 However, in Japan, clear cell carcinoma of the ovary represents more than 20% of all epithelial ovarian cancer.80 The prognosis of stage I clear cell carcinoma in Japan has been shown to be superior to that of serous ovarian cancer.
Clinical Manifestations, Patient Evaluation, and Staging
Patient Evaluation and Diagnosis
Serum CA 125 is a tumor marker that is elevated in 80% of women with epithelial ovarian cancer. It is predominantly correlated with serous ovarian cancer and is seldom high in cases of mucinous subtype. In postmenopausal women an elevated CA 125 higher than 65 U/mL was found to have a positive predictive value of 98% and a negative predictive value of 72%.81
Jacobs and associates82 proposed the “Risk of Malignancy Index” in 1990, estimating the risk of an adnexal mass being malignant in a specific patient. It has since been validated in many studies. The “Risk of Malignancy Index” incorporates the serum CA 125 level, ultrasound scan result (0, 1, or 3), and the menopausal state (0, premenopausal, 3, postmenopausal). These researchers defined a cutoff of 200 in which a sensitivity of 85% and a specificity of 97% for predicting malignancy were found.
Surgical Staging
Ovarian cancer is staged surgically according to the FIGO staging system, which was revised in 1995. The patterns of spread and natural history of ovarian cancer are the basis for the rationale behind this staging system27,83 (Table 59-1). The stage can be determined only after an exploration of the abdomen and pelvis, apart from the rare occasion in which stage IV disease may be documented by positive pleural effusion, fine-needle aspiration of a supraclavicular node, or a liver metastasis. Comprehensive surgical staging is pertinent, because the extent of disease will further dictate the need for subsequent treatment. In patients in whom no macroscopic disease is seen outside the ovary after careful inspection and palpation, an evaluation for microscopic disease must be performed. On entering the abdominal cavity, aspiration of ascites or peritoneal washings are to be performed. The abdomen and pelvis should be thoroughly examined and palpated; any suspicious lesions should be sampled. The tumor should be removed intact if possible, because spillage of malignant cells is suggested to worsen the prognosis.84 The remaining ovary, uterus, and fallopian tubes should be removed unless fertility sparing is appropriate (premenopausal patient with a low-grade or borderline ovarian cancer). Pelvic and para-aortic lymph nodes should be systematically removed, an omentectomy performed, and random peritoneal biopsy specimens taken. Carnino and colleagues showed that the detection of positive lymph nodes correlated with the number of lymph nodes removed (odds ratio [OR] = 3.9 for >10 lymph nodes in comparison to 1 to 5, 95% confidence interval [CI] = 1.0 to 15.4), therefore emphasizing the importance of systematic lymph node dissection.85 Patients initially thought to have early-stage disease that undergo subsequent comprehensive surgical staging may be upstaged in up to 30% of the cases.86
TABLE 59-1 FIGO Staging for Carcinoma of the Ovary
| Stage | Description |
|---|---|
| I | Growth limited to the ovaries |
| IA | Growth limited to one ovary; no ascites containing malignant cells. No tumor on the external surface; capsule intact |
| IB | Growth limited to both ovaries; no ascites containing malignant cells. No tumor on the external surface; capsule intact |
| IC | Tumor either stage IA or IB but with tumor on the surface of one or both of the ovaries; or with capsule(s) ruptured; or with ascites present containing malignant cells or with positive peritoneal washings |
| II | Growth involving one or both ovaries with pelvic extension |
| IIA | Extension and/or metastases to the uterus and/or fallopian tubes |
| IIB | Extension to other pelvic tissues |
| IIC | Tumor either stage IIA or IIB but with tumor on the surface of one or both of the ovaries; or with capsule(s) ruptured; or with ascites present containing malignant cells or with positive peritoneal washings |
| III | Tumor involving one or both ovaries with peritoneal implants outside the pelvis and/or positive retroperitoneal or inguinal nodes. Superficial liver metastases equals stage III. Tumor is limited to the true pelvis, but with histologically proven malignant extension to small bowel or omentum. |
| IIIA | Tumor grossly limited to the true pelvis with negative nodes but with histologically confirmed microscopic seeding of abdominal peritoneal surfaces |
| IIIB | Tumor of one or both ovaries with histologically confirmed implants of abdominal peritoneal surfaces, none >2 cm in diameter. Nodes negative. |
| IIIC | Abdominal implants >2 cm in diameter or positive retroperitoneal or inguinal lymph nodes or both |
| IV | Growth involving one or both ovaries with distant metastases. If pleural effusion is present, there must be positive cytologic test results to allot a case to stage IV. Parenchymal liver metastasis equals stage IV. |
It has also been shown that it is important that a gynecologic oncologist perform the operation. The percent of properly staged women varies by surgeon and is 97%, 52%, and 35% when the staging was performed by a gynecologic oncologist, obstetrician/gynecologist, or general surgeon, respectively.87
PRIMARY Treatment of Ovarian Epithelial Cancer
Early-Stage Disease
Two randomized trials—International Collaborative Ovarian Neoplasm Trial 1 (ICON1) and Adjuvant ChemoTherapy In Ovarian Neoplasm Trial (ACTION)88,89—addressed the question of the efficacy of adjuvant chemotherapy to improve survival in women with early-stage disease. Both trials randomized patients with early-stage disease to adjuvant platinum-based chemotherapy or no adjuvant treatment. The analysis of the combined trials showed an 8% difference in OS in favor of the adjuvant chemotherapy arm (82% vs. 74%, p = .008). Relapse-free survival (RFS) was also significantly better in the chemotherapy arm of the trials (76% vs. 65%, p = .001).
A literature review analyzed the results of 22 prospective randomized trials on the topic of benefit of adjuvant chemotherapy in patients who had optimal surgical staging of their disease.90 They concluded that adjuvant therapy is not advocated for patients with stage IA grade 1 tumors because their prognosis is excellent.91 It also is not beneficial for patients with early-stage, higher-grade disease.
Fertility-Sparing Therapy
Epithelial ovarian cancer occurs infrequently in women of childbearing age, with an estimated 8% of all malignant stage I epithelial cancers diagnosed in women younger than the age of 35.92 Although there is relative consensus in regard to conservative surgical treatment (i.e., adnexectomy with comprehensive surgical staging), for patients with early-stage tumors of low malignant potential the literature is limited in regard to young patients with invasive epithelial tumors. Schilder and associates,93,94 in 2002, published a multi-institutional study on 52 women with stage IA (n = 42) or stage IC (n = 10) patients treated with fertility-sparing surgery. Although all histologic cell types and grades were included, the majority of cases were mucinous (n = 25) and grade 1 (n = 38), and only 10 were of the serous cell type. All patients underwent surgical staging, including pelvic/para-aortic lymph node sampling, peritoneal biopsies, washings, and a partial omentectomy. Twenty patients received adjuvant chemotherapy. All patients with stage IC grade 2 to grade 3 received adjuvant chemotherapy. Five patients experienced relapse, and 2 died of their disease. The estimated 5-year OS was 98%, and the 10-year OS was 93%. Twenty-four patients, 6 who had received adjuvant chemotherapy, attempted to conceive and 17 succeeded (71%).
Radiation Therapy
The benefit of adjuvant RT in patients with early-stage epithelial ovarian cancer has been evaluated in a series of studies.95,96,97 Both intraperitoneal (IP) radioactive chromic phosphate suspension (32P) and whole-abdomen irradiation (WAI) with a boost to the pelvis have been evaluated. Recent series have suggested that the addition of RT to chemotherapy for subsets of patients may have an expanding indication.98–100
Intraperitoneal Radioactive Chromic Phosphate Suspension (32P)
Intraperitoneal instillation of radioisotopes such as gold (198Au) and chromic phosphate (32P) have been studied in patients with ovarian cancer. Although the initial studies were performed using 198Au, 32P became the preferred isotope because of ease of handling, lack of gamma radiation, and a relatively low complication rate.101 32P is a high-energy pure beta-particle emitter with a maximum energy of 1.71 MeV, a maximum penetration of 8 mm (average: 1 to 4 mm), and a half-life of 14.3 days. It can be used for intracavitary instillation at a dose of 10 to 20 mCi. The majority of the 32P is absorbed by the peritoneal surface via macrophages and then excreted through the lymphatic channels of the abdomen, that is, the thoracic lymphatics, and from there into the systemic vasculature where it is cleared by the liver. The retroperitoneal pelvic and para-aortic lymph nodes receive very low doses of radiation. Because the main pattern of spread of ovarian cancer is transcoelomic, the IP instillation of radiocolloids, which deliver high doses of radiation to the peritoneal surfaces while minimizing systemic effect, would seem ideal. Gamma camera imaging of the abdomen has shown that the administration procedure including mobilization of the patients in the first 6 hours is critical for homogeneous dispersion of the radiocolloids on the serosal surfaces.102 Dose calculations gave an estimated tissue surface dose of 30 Gy per 370 MBq of 32P administered. The amount of 32P in peripheral blood increased for 7 days after administration and was then followed by a continuous decrease. The estimated peripheral blood dose is 0.012 Gy, and the maximum bone marrow dose is 0.06 Gy.
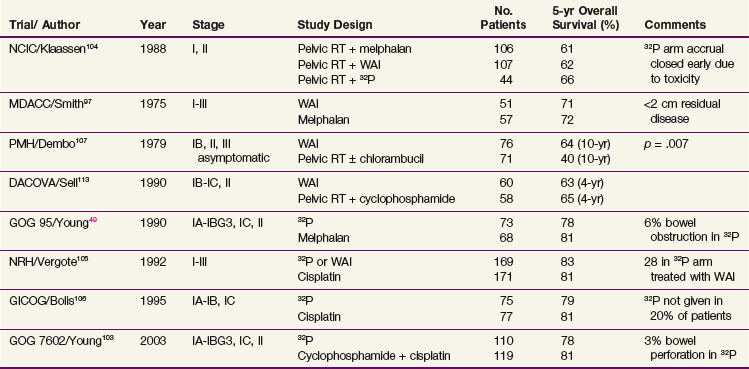
Several randomized studies evaluating the efficacy of 32P in early-stage ovarian cancer have been published49,103–106 (Table 53-2). The earliest of the randomized trials was published in 1988 by the National Cancer Institute of Canada (NCIC).104 Two hundred fifty-seven women with stage I to IIA high-risk ovarian cancer or with stage II disease were randomized to one of three trial arms: arm A, WAI (22.5 Gy in 20 fractions); arm B, melphalan; or arm C, 32P. Surgical staging was not mandatory, and most patients’ disease was not thoroughly staged. All patients were initially treated with pelvic RT (arm A: 22.5 Gy in 10 fractions; arms B and C: 45 Gy in 20 fractions). The 32P trial arm was discontinued early owing to increased bowel toxicity. No significant difference in survival was found.
In 1990 the Gynecologic Oncology Group (GOG) published a randomized controlled trial including 141 patients with stage I, grade 3 or stage II epithelial ovarian cancer randomly assigned to melphalan or a single dose of 32P at the time of surgery.49 With a median follow-up of more than 6 years the outcomes for the two treatment groups were similar with respect to 5-year disease-free survival (DFS) (80% in both groups) and 5-year OS (81% with melphalan vs. 78% with 32P; p = .48). They concluded that either treatment was a reasonable option.
In 1992, Vergote and colleagues105 published a randomized trial of 347 patients, stages I to III (no gross residual tumor), who were allocated to cisplatin (50 mg/m2) every 3 weeks for six cycles or 32P. Patients with intra-abdominal adhesions allocated to IP administration of 32P were treated with WAI (22 Gy to the whole abdomen in 20 fractions) followed by 22 Gy in 11 fractions to the pelvis. Patients did not undergo comprehensive staging, because lymph node sampling was not performed. The estimated 5-year OS of patients with stage I disease were 82% for the 32P arm, 94% for the WAI arm, and 79% for the cisplatin arm (p = .79, p = 0.44 compared with the cisplatin arm, respectively). Bowel obstruction occurred more frequently in those treated with 32P (9%, p = .02) or WAI (21%, p = .001) in comparison with the cisplatin arm (2%). Because no survival benefit was shown, cisplatin was recommended as the standard treatment.
The Italian Gruppo Interregionale Collaborativo in Ginecologia Oncologica (GICOG) performed a randomized trial106 of 152 women with stage IA grade 2, IB grade 2, and IC disease, comparing treatment with cisplatin with that with 32P. Patients underwent a surgical procedure that did not include lymph node sampling. In 15 patients randomized to treatment with 32P, the radioisotope was not delivered, owing to the development of abdominal adhesions. An improved 5-year PFS was reported for the cisplatin group in comparison with the 32P group (85% vs. 65%, p = .008) but without an OS difference (81% vs. 79%, p = .35).
The latest randomized study was published by the GOG in 2003. Two hundred twenty-nine women with early-stage ovarian cancer at high risk for relapse (defined as stage IA or IB grade 3, stage IC, or stage II, no macroscopic residual) were assigned to treatment with 32P or with cyclophosphamide and cisplatin.103 The cumulative incidence of relapse at 10 years was 35% for the 32P arm and 28% for the cyclophosphamide-cisplatin arm. Patients receiving cyclophosphamide-cisplatin had a relapse rate 29% lower than those in the RT arm (p = .15) and a death rate 17% lower than those in the RT arm (p = .43). The toxicity profiles of both regimens were reasonably well tolerated, although 3% of the patients receiving 32P suffered perforation of the small bowel during the insertion of the IP catheter and 7% had problems with inadequate distribution. Grade 3 or 4 gastrointestinal toxicity was seen in 12% of patients treated with cyclophosphamide and cisplatin. The authors concluded that, although there were no statistically significant differences, the lower relapse rate seen in the chemotherapy arm together with 32P complications make platinum-based chemotherapy the preferred adjuvant therapy.
External Beam Irradiation
Multiple randomized trials have been published evaluating the efficacy and toxicity of WAI in patients with early-stage ovarian cancer (see Table 59-2). Early studies established the inadequacy of treating the pelvis alone in patients with no gross residual disease.107,108,109
A trial from the Princess Margaret Hospital randomized 147 women with stage I to III disease comparing WAI and pelvic RT to pelvic RT alone or in combination with chlorambucil.107 After a 7-year follow-up, the 10-year OS difference was significantly higher in the 76 patients treated with pelvic and WAI in comparison to the 71 patients treated with pelvic RT and chlorambucil (46% vs. 31%, p = .05). The survival benefit was present only in patients with no gross residual disease or small volume residual (<2 cm).
Hreshchyshyn and associates110 published a randomized trial of 86 stage I (not surgically staged) patients randomized to pelvic RT, melphalan, or no additional treatment. They did not find a survival difference between observation and pelvic RT but did find a benefit for melphalan.
Several studies have compared chemotherapy to RT, although only one incorporated a cisplatin-based regimen.104,111–113 The NCIC randomized 257 women with stage I, IIA high-risk ovarian cancer, or stage IIB, and stage III to one of three trial arms: whole-abdomen/pelvic RT (22.5 Gy in 20 fractions), melphalan, or 32P.104 No significant difference in survival was found. Smith and associates97 randomly assigned 149 patients with early-stage ovarian cancer to postoperative whole-abdomen/pelvic RT or melphalan. No survival difference was found between the groups.
The Northwest Oncologic Cooperative Group of Italy (NOCGI) conducted the only prospective randomized trial112 in women with high-risk early-stage (stage I or II) ovarian cancer, comparing WAI with a cisplatin-based regimen. They randomized 70 women to cisplatin 50 mg/m2 and cyclophosphamide 600 mg/m2 or to WAI in an open technique, consisting of 43.2 Gy/24 fractions to the pelvis and 30.2 Gy to the upper abdomen. The study was closed early owing to multiple protocol violations (63% of patients were treated with chemotherapy) and low accrual. With a median follow-up of 60 months, the 5-year OS was 71% for the cisplatin and cyclophosphamide arm and 53% for the WAI arm (p = .16). Although toxicity was well tolerated in the chemotherapy arm of the study, in the WAI arm 28% of patients suffered from grade 3 to grade 4 diarrhea, 2 had severe enteritis requiring hospitalization, and 1 had late bowel obstruction requiring surgery.
In the majority of studies looking at the efficacy of RT, all epithelial malignancies were included. There is some suggestion that certain histologic subtypes might benefit from the addition of RT. Nagai and associates99 compared adjuvant platinum-based chemotherapy to WAI in 28 women with stage I to III ovarian clear cell carcinoma (CCC). Five-year OS and DFS in the RT arm were 81.8% and 81.2% versus 33.3% and 25% in the chemotherapy arm (p = .031, p = .006), respectively. Locoregional control was also considerably improved with adjuvant RT. Only 1 of the 16 patients receiving RT had an isolated locoregional recurrence versus 7 who had locoregional recurrences in the 12 patients in the chemotherapy arm. Dinniwell and associates98 performed a prospective, risk-stratified study of 29 patients with stage I to III epithelial ovarian cancer combining surgery, chemotherapy, and adjuvant WAI. The subset of 11 patients with clear cell carcinoma and 5 with endometrioid histologies showed the greatest gains from this aggressive, multimodality approach, with a 4-year DFS of 77% compared with 27% for serous subtypes (p = .01). Finally, a recent article by Swenerton and associates notes that patients with stage I and II clear cell, endometrioid, and mucinous histotypes had an improved survival with the addition of adjuvant WAI to chemotherapy.100 In these histotypes it may be reasonable to consider a combination of chemotherapy and RT. Given the concern over toxicity, and a suggestion of increased locoregional recurrences in these patients, there may be a role for adjuvant pelvic RT. At one of our institutions these patients are offered adjuvant pelvic RT after completion of chemotherapy.