Patient
Age
Sex
Primary
HIPEC
PCI
CC
FU
Surv
1
72
M
Sarcoma
Oxal
20
1
DOD
12
2
77
F
Sarcoma
Oxal
16
0
AWD
11
3
61
M
Sarcoma
Oxal
14
1
AWD
9
4
68
F
Small bowel
CDDP
26
0
QDF
23
5
51
M
Small bowel
CDDP
15
0
AWD
23
6
59
M
Small bowel
Oxal
20
1
AWD
8
7
46
F
Small bowel
Oxal
7
0
AWD
3
8
67
M
Pancreas
Oxal
23
1
ADF
5
9
67
M
Pancreas
Oxal
22
2
AWD
4
10
74
F
Pancreas
Oxal
3
0
ADF
8
11
70
F
GIST
CDDP
6
0
ADF
34
12
53
F
GIST
CDDP
12
0
ADF
108
13
73
M
GIST
CDDP
20
0
DOD
38
14
58
F
Breast IDC
CDDP 75 m
15
0
ADF
128
15
54
F
Breast ILC
CDDP
22
1
ADF
74
16
55
F
Breast ILC
CDDP
22
2
DOD
56
17
77
F
Breast IDC
CDDP
24
1
ADF
45
18
53
F
Breast IDC
CDDP
18
0
ADF
13
19
6
M
Bladder
CDDP
19
2
DOD
9
20
68
F
Uterus ADC
CDDP
5
0
DOD
46
21
56
F
Uterus ADC
CDDP
6
0
DOD
24
22
64
F
Uterus ADC
CDDP
23
0
AWD
12
23
58
F
Uterus ADC
CDDP
17
0
AWD
52
24
61
F
Uterus ADC
CDDP
9
0
ADF
95
25
67
F
Uterus ADC
CDDP
30
1
DOD
15
26
59
F
Uterus ADC
CDDP
29
1
DOD
15
27
65
F
Uterus ADC
CDDP
19
0
DOD
12
28
51
M
Lung
CDDP
19
0
DOD
7
Our study provides previously unavailable information on treating women with PM from BC. In our patients, a median of 18 years (range 10–30) elapsed after BC was diagnosed and PC developed, which accords with previous reports describing breast carcinoma as one of the most slowly growing solid tumors given that metastases may appear many years, even decades, after the initial diagnosis [22, 29]. Of the five patients treated, four achieved long-term survival, with one of them surviving for 10 years.
In patients with PC and an ovarian mass and history reporting previous BC, reaching a correct diagnosis can be difficult but is essential. As reported by other authors [30], immunohistochemical staining showing combined negative wild-type 1 (WT1) and cancer antigen (CA-125) tumor expression associated with positive GCDFP-15 expression in peritoneal disease invariably strengthened the diagnosis.
Our study extends current knowledge, showing that once the correct diagnosis is established, these patients can benefit from treatment. The study also possibly argues against previous reports describing poor prognosis. After maximal cytoreduction plus HIPEC, morbidity and mortality rates in our patients were in line with those reported for similar procedures. This combined treatment allowed good survival and quality of life (QOL). Although maximal cytoreduction plus HIPEC cannot be proposed as standard care for patients with PM from primary BC, survival rates observed in our small series suggest that in highly selected patients with no extraperitoneal disease and in whom surgery can achieve adequate cytoreduction, this combined procedure can offer patients with PC from BC a promising approach for long-term survival. This finding merits further investigation in larger studies.
22.3 Managing Peritoneal Carcinomatosis from Small-bowel Adenocarcinoma
Management strategy for patients with PM from small-bowel adenocarcinoma is unclear, and literature reports are episodic, even though PM is a frequent manifestation of small-bowel carcinoma [31]. Typically, these tumors present after a significant delay in diagnosis due to symptom vagueness and imaging difficulty, leading to poor prognosis and survival rates varying from 10 to 40 months. Marchettini and Sugarbaker [32] reported a median survival of 12 months in two of their patients treated with CRS plus HIPEC, with prolonged survival of 57 and 59 months, respectively. Chua et al. [33] published a review of seven patients treated with CRS plus HIPEC [mitomycin C and early postoperative intraperitoneal chemotherapy (EPIC) with 5-fluorouracil (FU)], reporting a median disease-free survival (DFS) of 12 months. They also reported a Kaplan-Meier analysis for a combined group of 19 patients treated with CRS plus HIPEC with a median overall survival (OS) of 29 months. Shen et al. [34] reported a median OS of 45 months after treatment with CRS plus HIPEC. A large, multi-institutional experience is reported by the French Association of Surgery [35], with a median OS for patients treated by CRS plus HIPEC of 32 months. In the four patients treated in our institution, one, who presented with intestinal obstruction, had a mean PCI of 17 (range 7–26). Mean OS was 31.2 months, with two patients alive and disease free at 43 and 22 months, respectively, and two alive with disease at 33 (pulmonary metastases) and at 27 (abdominal recurrence) months, respectively. The rarity of small-bowel carcinoma makes impossible the design of prospective trials. However, all series reported show better results compared with conventional treatments. Moreover, it must be considered that CRS plus HIPEC could represent the only valid surgical option for palliation in obstructed patients in whom a simple surgical procedure aimed at bowel decompression is often impossible due to small-bowel mesentery retraction or in those with associated ascites. Although it is impossible to conclude that CRS plus HIPEC is a treatment option for patients with PM from small-bowel adenocarcinoma, this combined treatment modality should be considered as a valid alternative in selected patients.
22.4 Managing Peritoneal Carcinomatosis from Endometrial Cancer
Endometrial cancer remains the most common cancer of the female reproductive tract. Treatment is surgery alone or in combination with brachytherapy and/or radiotherapy. Survival rates are approximately 90% at 5 years [36]. However, in cases of PM, patient management becomes more complex and prognosis is poor, with a median survival < 1 year. Bakrin et al. [37] reported on five patients with endometrial cancer treated by this combined modality, with a median survival of 19.4 months. Two patients experienced recurrent disease and died; three patients were alive and disease free at 7, 23, and 39 months, respectively after treatment. Glehen et al. [38], in a multi-institutional review of the French Surgical Association of 1,290 patients with PM from various primary tumors, reported in 2010 the treatment of 17 patients with uterine adenocarcinoma (13) and epidermoid carcinoma (four); however, their report did not provide specific survival data for this specific group of patients. Delotte et al. [36], in 2014, reported CRS plus HIPEC treatment in 13 patients with endometrial cancer. Five patients died of disease, three were alive with disease at 14, 26, and 28 months, and four were alive and disease free at 1, 60, 60, and 124 months, respectively. In our institution, from 2002, we treated eight patients with a diagnosis of uterine adenocarcinoma using CRS plus HIPEC. Mean PCI was 16, and complete cytoreduction was achieved in five patients; three patients had residual CC1 disease. In four patients, we observed recurrent disease: two died of disease at 9 and 13 months; two were alive with disease at 19 and 26 months; four were alive and disease free at 9, 14, 26, and 33 months. Treatment strategies for stage IV endometrial cancer remain controversial. Some reports highlight the histologic characteristics and extent of the disease as the main prognostic determinants; others favor the effects of a more aggressive surgical cytoreduction. Long-term survival reported in these observational studies were higher compared with those reported in the literature using conventional treatments, which seems to justify a more aggressive surgical approach with the aim of leaving patients without residual visible disease. CRS plus HIPEC could therefore represent a valid alternative to more conservative treatments, and survival results seem to justify future randomized trials.
22.5 Managing Peritoneal Carcinomatosis from other Unconventional Miscellaneous Tumors
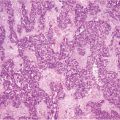
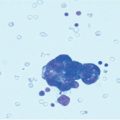
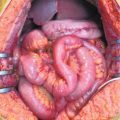
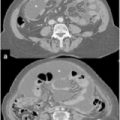
The increased interest in and reports of increased survival in treating PM with CRS plus HIPEC led many specialized centers to treat rarer and unusual primary tumors metastatic to the abdominal cavity and for which there no clear and accepted indications. The optimal management of these patients is a matter of intense debate. Systemic chemotherapy for PM has improved but remains limited because of poor drug diffusion into the peritoneum; however, the frequent tumor localization within the peritoneal cavity makes an appealing therapeutic option for selected cases. This is why many authors [1, 28, 34, 39–46] report small observational series of patients with PM from various unconventional tumors treated by CRS plus HIPEC (Table 22.1). This combined treatment modality has been used in PM from pancreatic, gastrointestinal stromal tumor (GIST), abdominal sarcomas, and gallbladder, liver, cholangiocarcinoma, adrenal, urachal, esophageal, and kidney tumors. In a multi-institutional review of the French Surgical Association on 1,290 cases of PM from various primary tumors treated with CRS plus HIPEC [38], there were 29 unconventional indications. Mortality was 4.1%, with a rate of major (grades 3 and 4) complications of 33%, similar to those reported after other major surgical procedures. Obviously, the numbers are too small to draw conclusion on survival figures for each specific primary tumor, but an overall median survival of 34 months, with a 5-year DFS of 22% compares favorably with survival figures reported in the literature regarding palliative treatment for the same tumor types.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree