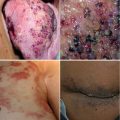
Fig. 12.1
(a) Kaposiform hemangioendothelioma (KHE) at the lower back in a 1.5-year-old boy. The red lesion was continuously growing during the first year of life, and biopsy was taken that confirmed KHE. (b) MRI with contrast enhancement of the cutaneous KHE. Arrow shows infiltration of the subcutis, but not the muscles or bones
The potential side effects of corticosteroid therapy include hypertension, gastritis, and delayed growth as well as mood changes. A Cushing’s syndrome with additional pituitary-adrenal axis suppression, secondary diabetes mellitus, or immunosuppression with increased susceptibility to infection is extremely rare. During corticosteroid therapy, the child should have a physical examination every 2–4 weeks with monitoring of blood pressure, glucosuria, as well as height and weight. Prescription of oral ranitidine or a beta-blocker may be necessary. However, all side effects, especially the growth and weight retardation, are reversible [7]. Live attenuated vaccines are contraindicated during the period of corticosteroid treatment, while vaccinations with dead vaccines can be performed once the initial high dosage of steroids is reduced.
Vincristine
There are very limited reports on the use of vincristine for the treatment of proliferating, corticosteroid- and/or interferon-resistant IH [8]. However, since the introduction of propranolol, vincristine is wildly more used for function- and life-threatening congenital vascular tumors [9].
In combination with corticosteroids, vincristine can be helpful in the Kasabach-Merritt phenomenon (KMP) that often is observed in the presence of congenital vascular tumors [10]. This phenomenon is defined as thrombocytopenia and hypofibrinogenemia with elevated fibrin split products (D-dimers), suggestive of an active consumptive coagulopathy. Thrombocytes are low, ranging from 6,000 to 98,000, with fibrinogen levels less than 100 mg/dL, whereas D-dimers are elevated. Prothrombin times (PT) and activated partial thromboplastin time (PTT) can range from normal to significantly prolonged. Additionally, anemia can be present at diagnosis as a consequence of intravascular hemolysis, including red blood cell fragmentation, elevated LDH, and hyperbilirubinemia. Successful treatment of the underlying vascular tumor is critical to the correction of KMP and to the overall survival of patients. Children with KMP can die of hemorrhage or invasion/compression of vital structures by the vascular tumor. Mortality has ranged from 10 to 30 % in most series. A curative therapy of KMP can only be achieved by treatment of the underlying vascular tumor. However, supportive care to maintain hemostasis is necessary. Platelet transfusions should be reserved for active bleeding or in preparation for surgery or procedures. Aminocaproic acid and local measures may be helpful to reduce the need for platelet transfusions. Antiplatelet agents, such as acetylsalicylic acid and dipyridamole, have been used to reduce platelet aggregation within the vascular tumor. Treatment of hypofibrinogenemia with cryoprecipitate and prolonged PT or PTT with fresh frozen plasma should be a clinical decision rather than correction of a laboratory result. Symptomatic anemia should be treated with red blood cell transfusions. A retrospective study on 15 patients with different vascular lesions and KMP shows that vincristine can be a safe and effective drug not only to treat KMP but also to decrease the size of the vascular tumor [10]. A recently developed guideline on the management of KMP supports the combination of corticosteroids and vincristine as the first-line therapy for KMP [11]. We present the MRI of a 9-months-old child with severe thrombocytopenia and anemia. Bone marrow morphology was normal and further diagnostics revealed a congenital vascular tumor at the retroperitoneum (Fig. 12.2 ). Therapy with corticosteroids and vincristine induced remission of KMP as well as shrinkage of the vascular tumor that could not be biopsied because of danger of bleeding.
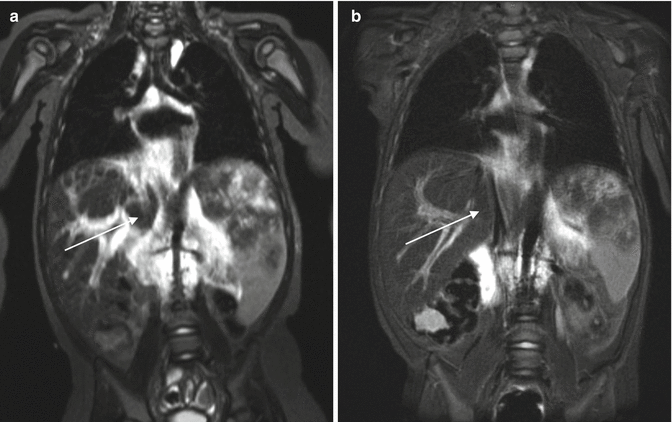

Fig. 12.2
(a) MRI of a 9-month-old child with a congenital vascular tumor of the retroperitoneum. (b) MRI 6 months after corticosteroids and vincristine therapy. Arrows in (a) and (b) show a diffuse lesion which has shrinked during therapy
Vincristine is a naturally occurring vinca alkaloid isolated from the leaves of the periwinkle plant Catharanthus roseus [12]. It interferes with mitotic spindle microtubules by binding to tubulin, resulting in inhibition of mitosis. There is considerable experience with the use of vincristine in the treatment of malignancies in children. Vincristine is a vesicant and caution needs to be exercised if given peripherally due to the risk of extravasation. Vincristine can be administered at a dose of 1 mg/m2 (or 0.05 mg/kg body weight in children <10 kg). It is generally used intravenously with weekly injections first and then tapering down by increasing the interval between injections, depending on the clinical response [13].
Neurotoxicity is the dose-limiting side effect of vincristine. A peripheral mixed sensory and motor neuropathy is common. It can also produce an autonomic neuropathy resulting in abdominal pain, constipation, and ileus. Hematologic toxicity is rarely encountered with vincristine.
Interferon
The use of interferon alpha subcutaneously at a dose of 1–3 × 106 million units/m2 body surface area daily has been widely reported for corticosteroid-resistant proliferating IH in the literature [14]. Because of its dangerous side effects and the high effectiveness of propranolol, the use of interferon alpha for the therapy of IH has been abandoned. Acute toxicity includes fever, neutropenia, and anemia. Of great concern is the long-term neurotoxicity with development of spastic diplegia in up to 20 % of treated IH [15]. Interferon alpha was initially developed as an antiviral agent and has immunoregulatory, antineoplastic, and anti-angiogenic properties [16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree