41.1
Introduction
As a comorbidity of neurological disease, osteoporosis (OP) is often underdiagnosed, undertreated, or totally ignored in the face of more compelling circumstances that require immediate and continuous attention. Encompassing more than 600 different diseases, neurological conditions affect both the central and peripheral nervous systems. The three neurologic disorders considered in this chapter are among those with the highest fall rates that can predispose patients to disabling fractures and thereby further compromise their mobility. These conditions affect the brain, spinal cord, and a large network of nerves, neurons, and muscles, with progressive cell death resulting from neurodegenerative conditions in Parkinson’s disease (PD), rapid cell death in stroke, and damage to neural connections in multiple sclerosis (MS).
In terms of an association with metabolic bone disease, increasing evidence indicates that patients with neurological disease face a substantially greater risk of OP than do age-related controls. Yet, as indicated by the Global Longitudinal Study of OP in Women cohort study , patients in North America, Europe, and Australia who experience PD, stroke, and MS consistently underappreciate their fracture risk due to oversight by nonbone specialists, absence of appropriate fracture risk assessment tools and strategies, and lack of national and international recommendations to guide the management of OP associated with these disorders . The following outlines the risk factors, assessment tools, and treatment options for metabolic bone disease in three of the most burdensome neurological diseases worldwide.
41.2
Parkinson’s disease
PD is a chronic, progressive, and neurodegenerative disease characterized by motor symptoms such as tremor, muscular rigidity, bradykinesia (slowness, small movements, or the absence of movement), postural instability, loss of control over face movements, and nonmotor symptoms, including depression, anxiety, and cognitive decline . After Alzheimer’s disease, PD is currently the second most prevalent neurodegenerative disease in the United States and the world. The “Parkinson’s Prevalence Project,” released in 2018 and drawn on diverse population groups, estimates that by 2030, approximately 1,240,000 individuals in the United States will be living with the disease . Moreover, PD is now the fastest growing neurological disorder, with some experts projecting that it is on the verge of becoming a pandemic. A 2014 metaanalysis estimates that the number of people with PD will double from 6.9 million worldwide in 2015 to 14.2 million in 2040 .
41.2.1
Risk factors for osteoporosis in Parkinson’s disease
41.2.1.1
Low bone mineral density
A series of case–control studies of OP and osteopenia in PD demonstrates that lower bone mineral density (BMD) severely affects fracture risk, independent of falls. Using World Health Organization (WHO) criteria or the diagnosis of OP and focusing on relatively “young” PD patients (mean age=60.0±9.25 years), Bezza et al. found that BMD at lumbar spine and hip was lower among patients than age-matched controls, with more than half of a group of 52 men and women with low BMD . Reduced levels of BMD in the hip, lumbar spine, and femoral neck were subsequently confirmed by Zhao et al. and by Torsney et al. . In terms of the relative severity of bone loss, Torsney’s 2013 metaanalysis of 23 studies found BMD levels in PD to be −0.05, 95% confidence interval (CI) for total hip; −0.08, 95% CI for femoral neck, and −0.09, 95% CI for lumbar spine . However, a subsequent Chinese study based on T -scores found that bone loss in PD was most severe in the lumbar spine; results from other relevant studies are not consistent .
41.2.1.2
Age, gender, disease severity, and duration
Increasing age predisposes the population group more than 45 years not only to PD but also to OP. Indeed, a considerable number of individuals who experience PD may already have OP. The comparative risk of OP in males and females with PD has been disputed, but the prevailing consensus points to a higher risk for women, consistent with the overall predominance of OP in women in addition to overlying neurologic factors.
The relationship between PD severity and duration and the degree of OP has also been subject to varied interpretations. Lorefalt et al. and van den Bos found no correlation between disease severity and disease duration. Other studies revealed a direct correlation, but generally in association with confounding factors .
One of the key measures employed in these analyses is the Hoehn and Yahr (H&Y) scale that delineates the progress of PD through five stages, ranging from Stage I, marked by mild symptoms on one side of the body, to Stage III characterized by loss of balance and slowness of movement to the most advanced, Stage V, at which the patient is generally confined to bed or a wheelchair with full-time assistance required to perform daily activities and prevent falls. Dividing the patients into two groups, H&Y I/II and H&Y III–IV, measured against age, duration, site, and T / Z scores, Gao et al. summarized his results in Table 41.1 . The comparison of H&Y levels to the previous factors illustrates that OP becomes worse, as the severity and duration of the disease progress.
| H&Y I and II | H&Y III and IV | P | |
|---|---|---|---|
| Age | 64.31(6.63) | 65.01 (9.26) | .152 |
| Duration (years) | 3.25(2.55) | 4.12 (3.64) | .232 |
| L spine BMD | 0.845(0.11) | 0.75 (0.10) | .005 |
| L spine T -score | −1.94(0.88) | −3.01 (0.79) | .003 |
| L spine Z -score | −0.74(0.87) | −1.93 (0.98) | .002 |
| Fem neck BMD | 0.69(0.10) | 0.61 (0.10) | .027 |
| Fem neck T -score | −1.66(0.70) | −2.35 (0.78) | .015 |
| Fem neck Z -score | −0.36(0.59) | −1.13 (0.67) | .007 |
| Hip BMD | 0.86(0.08) | 0.77 (0.12) | .004 |
| Hip T -score | −0.93(0.46) | −1.64 (0.61) | .000 |
| Hip Z -score | −0.077(0.56) | −0.507 (0.55) | .001 |
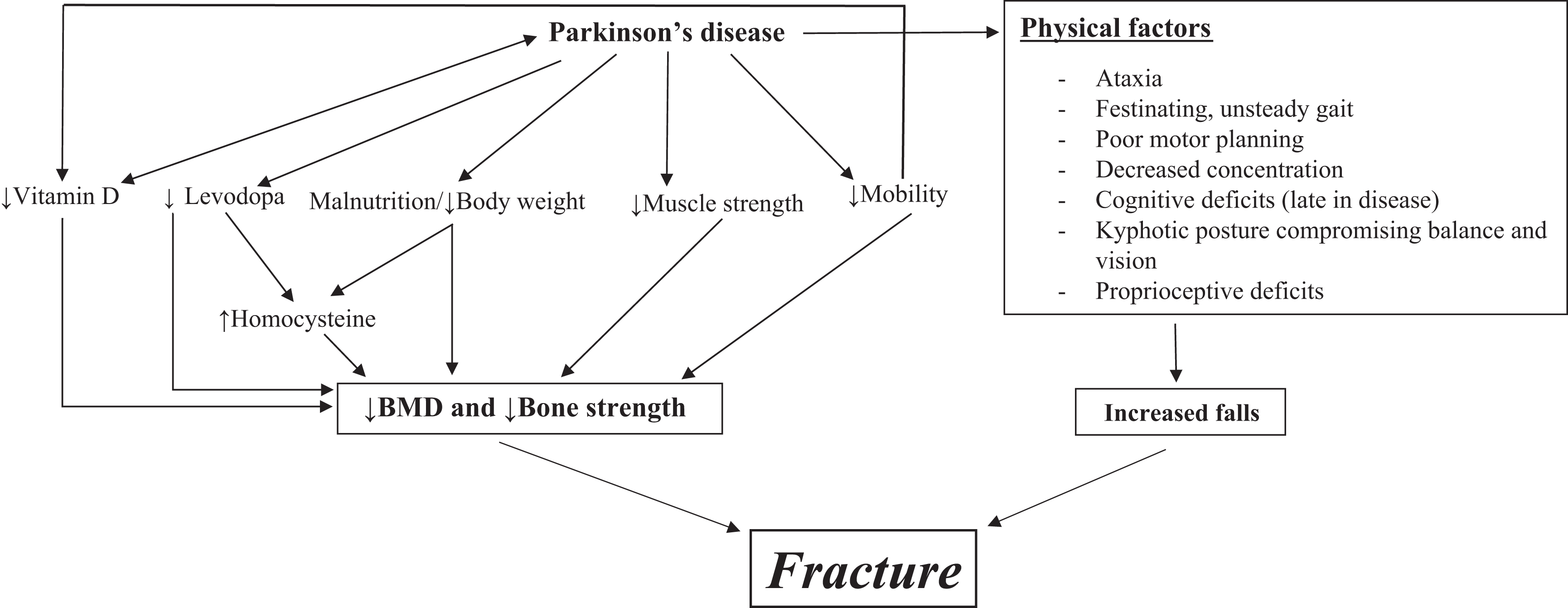
However, Gao, as well as other researchers supporting the severity/duration/OP correlation, points to confounding factors as well. For example, Abou-Raya et al. also took into account a physical and mental performance assessment, while Aithal et al. emphasized the association between advancing age, female gender, and duration and severity of PD to OP, with severity and long duration being the best single predictors. They also cite additional variables, including falls and fractures, restricted sunlight exposure, nutritional deficits, weight loss, physical inactivity, and treatment for PD such as levodopa, as discussed next. Among the circumstances that account for discrepancies in these studies are small study samples and differing inclusion criteria. Factors influencing BMD and loss of bone strength are illustrated in Fig. 41.1 .
41.2.1.3
Falls and fractures
Over the past 30 years, PD has emerged as the neurological disease most closely associated with falls, leading to fractures. As early as 1986, Johnell and Sernbo described a sixfold fracture risk in “patients with signs of Parkinsonism” . Further research has shown that 18.3% of individuals with PD experienced at least one injurious fall in an average of 20 years prior to their diagnosis, as opposed to 11.5% for controls. In addition, the risk of fractures in PD was 36% higher than in controls, at least 15 years before diagnosis, leading to the possibility that clinically relevant neurodegenerative impairment may be present decades before the clinical onset of PD .
In a systematic review of patients in which PD has developed, Allen et al. showed fall rates to be double the reported rate in community-dwelling elderly people without neurological disease. In patients monitored prospectively over 1–29 months, the rate is 60.5% (range of 35%–90%) for those reporting at least one fall and 39% (range of 18%–65%) for those reporting recurrent falls . More recent studies have dealt with frequency of falls in newly diagnosed Parkinson’s patients. In an investigation involving 181 drug-naïve PD subjects and controls, 64.1% of patients reported falling during 7 years of follow-up; older individuals at baseline had the greatest risk of falling . In a related study involving 99 newly diagnosed patients more than 54 months, the single fallers (7) were younger than the recurrent fallers (57) by almost 15 years .
In the United States, PD patients are almost twice as likely to experience falls and fractures compared with non-PD patients, independent of age and gender . A metaanalysis of 580,470 participants from 287 observational studies found that in comparison with healthy controls, PD was associated with a higher risk of fracture (OR=1.92, 95%, CI 1.72–2.15) as well as an increased risk of OP (OR=1.89, 95%, CI 1.30–2.75) .
Hip fractures are the most common type of fractures in PD. A 20-year nationwide sample of hip fractures in 20% of US hospital admissions found that patients with PD were overrepresented by a factor of four . One possible explanation for the prevalence of hip fractures is the fact that PD patients, with their characteristic shuffling gait and slower gait speed, tend to fall straight down, backward, or to the side, causing a direct impact on or near the hip .
However, fractures in PD can also occur at other sites associated with low BMD as well as low body fat, low body mass index, and high fall risk . Two recent Korean studies have focused on the risk of osteoporotic vertebral fractures (OVF) in PD. Comparing PD patients over the age of 60 with controls, Lee et al. reported that 12.5% of patients in the PD group developed OP vertebral compression fractures as opposed to 7.4% of those in the control group. Morality rates were 1.7 times higher in those with PD than in controls . A 2019 study focused specifically on the prevalence of OP and OVF in the Korean PD population, with reference to the socioeconomic status of the participants . Two large databases were used: one covering patients enrolled in Korea’s National Health Insurance Plan, covering some 97% of the population; the other covering the remaining 3%, serving those with a lower socioeconomic status who were enrolled in the country’s Medical Aid program, analogous to US Medicaid. The study reported significantly higher prevalence rates for OP (14%) and OVF (1.8%) in PD patients than in the general population (OP, 4%, OVF 0.5%). Even higher rates for those in the Medical Aid group indicating a potential link, but not a direct causal relationship, between low income and fracture risk . The study further reported that both OP and OVF increased with age and occurred with greater frequency in women. At the same time, Torsney et al. have warned that the prevalence of vertebral fractures in PD could be underestimated because the stooped position characteristic of the disease may deter clinical investigation of OP .
41.2.1.4
Vitamin D deficiency
A long-established risk factor for OP, vitamin D deficiency, related to immobilization and limited sun exposure, is a primary characteristic of PD. In a systematic review of six relevant studies focusing on the relationship between vitamin D and PD, Rimmelzwaan et al. determined that all but one investigation indicated that serum 25(OH)D in patients with PD was significantly lower than in age-matched healthy controls (significant mean difference of 11.6 ng/mL) in the PD group compared with controls. The fact that vitamin D deficiency is also significantly correlated with BMD of the hip and lumbar spine in PD underlines its role in the progression of bone loss .
41.2.2
Immobilization, reduced sunlight exposure, and hypercalcemia
Restricted daily living activities, limited exercise, and immobilization that are characteristics of PD pose a serious threat to bone health. In a study of disuse OP, Takata et al. point out that the resulting reduction of mechanical stress inhibits osteoblast-mediated bone formation while accelerating osteoclast bone resorption . The rate of bone loss that occurs is dependent on the duration and acuteness of the immobilization; a study of patients with early stage PD showed the greatest decrease in BMD in those with more advanced disease who evidenced further diminished activities, greater motor impairment, and less sunlight exposure .
Immobility restricts sun exposure that is the key source of vitamin D for every individual. It has long been known that solar UVB photons are absorbed by 7-dehydrocholesterol in the skin, leading to its transformation to previtamin D and rapid conversion to vitamin D3 . Neither a vitamin D–enriched diet nor the recommended supplement of 800–1200 IU of vitamin D daily is considered sufficient to provide adequate amounts of circulating 25(OH)D; estimates indicate that whereas food provides only 20% of vitamin D, ultraviolet radiation is the source of remaining 80%. However, vitamin D supplementation may indirectly enhance access to sunlight by improving balance and ambulation, thereby reducing falls and decreasing sway in elderly fallers. A study of 40 PD patients by Peterson et al. found that vitamin D supplementation reduced falls and decreased sway in elderly fallers .
In addition, immobility in PD can induce both hypercalcemia and hypercalciuria. Without the benefit of mechanical stress on bone, prolonged immobilization uncouples bone remodeling ; thus immobilized PD patients, particularly females over the age of 50, experience accelerated bone resorption and suppressed bone formation . Simply put, the weakened bones release more calcium into the blood, decreasing bone density and leading to OP, particularly of the cortical bone . Hypercalcemia also has other clinical manifestations, including neuromuscular effects, such as impaired concentration and muscle weakness, nausea and abdominal pain, excessive urination and dehydration, and kidney stones and kidney failure.
With respect to hypercalciuria, it has long been known that immobilized patients experience “resorptive” or bone-derived hypercalciuria, associated not only with increased calcium excretion but also with inhibited secretion of the parathyroid hormone (PTH) and, in turn, suppression of the PTH, 1–25-dihydroxy-vitamin D axis . Decreased PTH levels and reduced renal calcium absorption ultimately lead to hypercalciuria that, in turn, increases BMD loss as well as the risk of renal stone formation and impaired renal function. Moreover, there appears to be a correlation between hypercalciuria in calcium stone–forming patients and low bone volume, a tendency to low bone formation, increased bone resorption, and a severe mineralization defect, consistent with normal or low bone turnover OP .
41.2.2.1
Muscle weakness
Muscle weakness (decreased ability of the muscle to generate force) in PD is associated with a number of interrelated factors, ranging from immobility, bradykinesia, and vitamin D deficiency to poor nutrition and such nonmotor complications as depression, apathy, and a sedentary lifestyle. The resulting lack of physical activity further contributes to muscle weakness, creating what Speelman et al. have termed a “vicious circle” between physical inactivity and decreased muscle strength . At the same time, other researchers maintain that the core of muscle weakness in PD is related to dopaminergic deficits, citing how quickly anti-Parkinson’s medication and deep brain stimulation can improve movement speed .
The loss of muscle strength in PD is most evident in the upper and lower extremities, particularly in both hip and knee flexors and extensors , but it has also been detected in the trunk where hip BMD has been shown to be independently associated with leg muscle strength in ambulatory women with PD .
41.2.2.2
Weight loss/nutritional deficits
A known risk factor for low BMD, unintended weight loss has been frequently reported in PD patients, dating back to its inclusion in James Parkinson’s first publication on PD in 1817. In a study of weight loss, motor symptoms, and disease progression, Cersosimo et al. observed that weight loss was more prevalent and severe in PD patients than in controls, occurring in almost half of PD subjects and often beginning before motor symptoms are detected. Moreover, it is largely the consequence of disease progression rather than involuntary movements or a decrease in food intake. In addition, the conditions associated with PD, including poor hand-to-mouth coordination, dysphagia (difficulty in swallowing), gastrointestinal dysfunction, and cognitive impairment, all contribute to malnutrition and, in turn, to low levels of vitamin D, B12, folate, and elevated homocysteine (Hcy)—all have negative effects on bone formation and bone strength .
41.2.2.3
Effect of anti-Parkinsonism drugs
The concurrent use of multiple drugs and association with fracture risk is also a serious concern in PD. The folic acid and vitamin B12 deficiencies noted previously contribute to elevated serum Hcy concentration or hyperhomocysteinemia, as does levodopa, an effective dopamine replacement agent used in the treatment of PD for over 40 years . A 2010 study focusing on levodopa as a risk factor for OP found that higher serum Hcy concentrations were independently associated with higher fracture risk, and that patients taking higher doses of levodopa had significantly higher serum Hcy concentrations; in addition, greater levodopa intake is among the most important factors contributing to lower BMD . One of the mechanisms linking higher Hcy to low BMD is the interference of Hcy with collagen cross-linking, leading to poor bone quality coupled with lower bone mass. The results of this study clearly indicated that serum Hcy concentrations were independently associated with lower hip BMD and higher fracture risk.
In terms of other treatments for PD, Vestergaard et al. determined that dopamine agents, anticholinergic drugs, and monoamine oxidase B inhibitors are not associated with increased fracture risk, but that neuroleptics did result in an increased number of fractures in almost all skeletal sites and at all dosage levels .
41.2.3
Management strategies
Just as researchers are becoming increasingly aware of the risk for OP in PD, so physicians too must become increasingly aware of the need to include assessment of that risk in their diagnosis and subsequent treatment of the disease. In the absence of guidelines specifically related to management of OP in PD, recommendations set forth by the American College of Physicians and Surgeons and similar organizations for non-Parkinsonian patients should be consulted .
Whether FRAX and/or dual-energy Xray absorptiometry (DXA) imaging should be employed for all PD patients at risk of OP is subject to varied interpretations. Management assessment can range from FRAX employed with or without a BMD value for all PD patients to a more restrictive approach limiting assessment to PD patients with a history of falls, use of bilateral walking aids, disease duration of ≥5 years, or a previous osteoporotic fracture .
As an alternative tool, Lyell et al. have proposed a PD-specific algorithm to guide management of patients with H&Y Stages I–IV disease . Incorporated into the recently updated British OP guidelines accredited by the National Institute for Health Care and Excellence , it stipulates that for PD patients over age 70, a FRAX 10-year probability of 20% or more for major OP-related fractures (MOFs), or of 5% or more for hip fractures, should dictate the need for serial DXA imaging. If these thresholds are not reached, but MOF probability is over 11%, it is appropriate to obtain new or repeat BMD measurements and recalculate FRAX. These thresholds are lower for younger people but increase with age .
In considering these possibilities, it should be noted that comorbidities such as PD and MS are not included in FRAX, nor does FRAX take into account multiple clinical risk factors for secondary OP. However, the use of some form of fracture risk assessment is critical in determining whether to use either or both nonpharmacological and pharmacological therapy for OP in PD.
41.2.4
Treatment options
41.2.4.1
Nonpharmacological interventions
The various treatment strategies discussed here relate primarily to factors specific to OP in PD and do not include general management strategies that are also applicable to PD, such as smoking cessation and limiting alcohol consumption.
Fall prevention
Whereas a history of falls and disease severity for a given patient cannot be altered, many other interventions can be adopted to prevent falls in PD, including alleviating the freezing of gait, improving balance and mobility, increasing muscle strength, and modifying cognitive deficits, through the use of exercise or movement strategy training . Generally regarded as among the most severe symptoms of PD, gait freezing affects up to half of PD patients, broadly defined as the inability to begin stepping forward, it is caused by the interaction of motor, cognitive, and behavioral and is a major factor in precipitating falls . Cheng et al. have compiled a helpful consensus document offering guidance on 31 fall risk factors, both generic and specific to PD .
Exercise
In the past two decades an increasing number of studies have considered the effects of exercise, ranging from balance training and walking programs to muscle strengthening and stretching, on fall reduction in PD. A home-based exercise program, developed in 2007 by a physiotherapist and designed for PD patients with repeated falls, encompassed muscle strengthening, stretches, balance training, and cognitive movement strategies . While the program demonstrated a “consistent trend” of reduced fall rates among participants, the differences were insignificant. At the same time, rates of near falls and repeat near falls were significantly lower than for those in a control group, leading to the suggestion that individuals with less severe or earlier PD might benefit from a similar program. Ten years later, in a metaanalysis of 25 randomized controls trials examining balance and gait function in PD, Shen et al. found that facility-based training, supervised by physical therapists, resulted in greater improvement over the long term than did community- or home-based training. They also reported that the effect of exercise was positive in both the short- and long term, supporting earlier results by Tomlinson et al. and Allen et al. , demonstrating that exercise reduced the fall rates of PD patients by a significant 60% in both the short- and long term but had an insignificant effect on the number of fallers.
Interestingly, postural stability in patients with PD can be positively influenced by Tai Chi and even yoga . In mid-to-moderate PD a Tai Chi protocol, emphasizing rhythmic weight shifting and ankle sway and used twice weekly for 24 weeks, was more effective in improving postural stability and falls in comparison with resistance training and stretching programs. Moreover, Tai Chi also significantly reduced the incidence of falls, as compared with stretching but not with resistance training (which has notable drawbacks in terms of safety monitoring and equipment requirements). This and similar studies represent an advance in behavior-based treatments that can reduce fall incidence in PD.
A number of technology-based interventions for fall prediction and prevention, including wearable sensors, neuroimaging, and gait adaptability assessment, are under development but thus far, many have proved inconclusive with little direct application to PD.
Dietary supplementation
In the absence of specific studies on the effect of vitamin D supplementation on BMD and fractures in PD, physicians should recommend a healthy diet, including an average of 1200 mg calcium and 800–1000 IU of vitamin D daily, as advised by the National Osteoporosis Foundation, with adjustments determined on a case-by-case basis. Few high-quality studies of the effect of vitamin D on BMD or fractures in PD exist as of this writing . In a broader pooled analysis of the relationship between vitamin D dose requirements and fracture prevention (31,022 persons with a mean age of 76), Bischoff-Ferrari et al. reported a “somewhat favorable” fracture risk reduction only at the highest intake level of ≥800 IU daily, with a 30% reduction at the hip and 14% for any nonvertebral fracture . Again it should be noted that this finding is the result of a generalized study, not one pertaining specifically to PD. Furthermore, the role of high-dose vitamin D on fall prevention in all patients remains controversial at this time.
41.2.4.2
Pharmacological treatments
As indicated earlier, high Hcy levels exert a negative impact on BMD in PD patients taking levodopa. A study by Lee et al. demonstrated that Hcy-lowering therapy resulted in BMD increases of 4.4% at the lumbar spine and 2.8% at both the total femur and femur shaft, leading to their conclusion that Hcy therapy had the potential to become an inexpensive treatment for bone loss . With the naturally occurring antioxidant, alpha lipoic acid (ALA), they found significantly greater changes (4.6%) in BMD at the trochanter than in controls. New research on the effect of ALA more broadly has produced some promising results indicating that it may be effective as an antiosteoclastogenic agent. Data show that ALA is protective against bone loss by means of a two pronged mechanism, the first, inhibiting the generation of osteoclastogenic reactive oxygen and the second, upregulating redox gene expression . The results of these studies may be promising, but further controlled trials with larger populations are needed to determine whether the full potential of ALA can be realized in degenerative diseases such as PD.
Effects of catechol- O -methyltransferase inhibitors (entacapone, tolcapone, and tropolone) on BMD varied, some showing reduced plasma Hcy levels and others demonstrating no significant effect .
Bisphosphonates with their known side effects have been used to treat OP in Parkinson’s, but physicians must rely on data from studies of OP more broadly to judge their applicability to PD. No bisphosphonate interactions are known to occur with levodopa, dopamine agents, and other treatments for PD . However, uncontrolled movements and problems with swallowing in PD may make oral administration problematic. Zoledronic acid (ZA), an intravenous bisphosphonate generally administered once yearly, is FDA approved for the prevention and treatment of postmenopausal OP and for the prevention of fractures after hip fractures , but as of this writing, no studies of ZA in PD patients have been conducted.
The human monoclonal antibody, denosumab, inhibits the formation and activity of osteoclasts increasing BMD and reducing fractures. Administered every 6 months as a subcutaneous injection, its ability to increase cortical BMD may make it particularly suitable for PD, where hip fractures predominate . PTH and abaloparatide (Tymlos) are also possible alternative treatments for OP in PD.
In summary the growing prevalence of PD coupled with the multiplicity of risk factors for OP should be further investigated to gain a greater understanding of how they affect bone health in PD, individually and synergistically. It is recommended that appropriate risk-management strategies and DXA screening be employed as warranted. In the absence of guidelines specific to OP in PD, general guidelines for OP and relevant research studies should be implemented.
41.3
Stroke
Just as PD is implicated in the onset of OP, so too is there a relationship between stroke and OP. Unlike PD that has been experiencing a steadily increasing prevalence rate, stroke incidence, prevalence, and mortality has declined from 1990 to 2013 . However, the annual percentage change (APC) in stroke death rates showed a 2.5% increase per year from 2013 to 2015. This more recent trend includes a statistically significant increase among Hispanics (APC 5.8%) and people in the South Census region of the country (APC 4.2%). Declines in death rates are no longer observed in 38 states . At the same time, the overall global stroke burden, in terms of the absolute numbers of people disabled or otherwise affected by stroke, or who remain disabled from stroke, has increased in men and women of all ages . More people who have experienced a stroke are living longer, due primarily to improved stroke care and superior clinical management of modifiable risk factors. In 2015 stroke accounted for the largest proportion of disability-adjusted life years worldwide, amounting to 47.3% of the total, with deaths at 67.3% .
In the United States, more than 795,000 people experience a stroke each year; estimates indicate that by 2030, 3.88% of American adults, nearly 1 in every 25, will have experienced a stroke, representing a 20.5% increase from 2012 . Prevalence of stroke in the United States increases with advancing age in both males and females and, given an aging population, stroke prevalence will increase even further, especially among elderly females. As the National Stroke Association reports , only 10% of stroke survivors recover completely. Some 25% recover with minor impairments, while nearly half continue to live with serious impairments requiring specialized ongoing care. As was evident with PD, treatment and rehabilitation for stroke focus on alleviating the adverse effects of the primary disease, with OP either preceding stroke or occurring after an attack, receiving limited if any attention.
41.3.1
Occurrence of osteoporosis and its symptoms prior to stroke
One of the few studies of OP symptoms prior to the onset of a stroke, carried out by Kim et al., found that of 48 patients (28 female, mean age=68.9±8.7; 20 male, mean age=64.8±8.5) examined within the initial 30 days of a first stroke, 43.8% had established OP at the onset, while 39.6% were osteopenic . Of these 48 patients, 31.3% evidenced OP in the total hip, 39.8% were osteoporotic at the femoral neck, and 31.3% at the lumbar spine. Equally if not more significant, 25% had at least one thoracic or lumbar vertebral body (VB) fracture and 16.7% had two or more VB fractures. Studies implicating low BMD and hip fracture as predictive factors for stroke are extremely limited. An examination of the relationship between low BMD and stroke risk revealed that women, but not men, with BMD values in the lowest quartile had a higher risk of stroke than women with BMD values in the highest quartile, an association that remained significant even after the analysis was adjusted for potential confounders. Although the authors do not propose a cause and effect relationship between low BMD and high risk of stroke, they do assert that both conditions may be related to estrogen deficiency, hypertension, physical inactivity, and smoking. An investigation of the frequency and risk of stroke in the year following hip fracture revealed that patients with the fracture had a 50% higher risk of having a stroke in the subsequent 12 months, as opposed to comparable patients with no fractures . However, the cause of the heightened risk is unclear, and the fractures may be attributable to factors other than OP.
41.3.2
Risk factors for osteoporosis following stroke
Stroke and PD share many of the same risk factors for OP, including low BMD, physical inactivity and immobility, vitamin D deficiency, falls and fractures, and awkward body movements. Over the past quarter-century, numerous studies have elucidated the underlying causes of these risks, with repeated recommendations to institute OP screening early after stroke onset.
41.3.2.1
Reduced bone mineral density
As Huo et al. have observed, a two-way interaction exists between stroke and bone health . Whereas OP may be an independent risk factor for brain white matter changes and/or silent infarctions as detailed by Minn et al. , stroke contributes to low BMD that results in or exacerbates OP.
Loss of BMD begins within a few days after a stroke, with the most dramatic decrease occurring in the first 3–4 months by a mean of 9.3% and 3.7% in the upper and lower limbs, respectively . It then progresses more slowly until the end of the first year poststroke when it reaches a steady state. Research also shows that the paretic side is primarily affected by bone loss. The higher BMD values on the nonparetic side can be traced to increased weight-bearing input and greater physical activity , whereas the lower levels are due to reduced mobility leading to decreased skeletal loading and increased bone resorption. As Beaupre and Lew have observed, in some patients, bone loss in the paretic arm 1-year poststroke is the equivalent of >20 years of physiological bone loss in healthy individuals .
Bone loss in the upper extremities (humerus or distal radius) is greater by 12%–17% than in the lower extremities, which may experience either no change or even an increase in BMD . Progressive hemi-OP on the paretic side and increased BMD in the nonparetic arm occur within the first year after severe stroke .
A recent study in male stroke patients, aged 50–65 years, 3–4 months poststroke, determined that OP prevalence was 38.1%, significantly higher than controls at 16.5%, emphasizing the high level of bone loss in the months immediately following the stroke . During this period the decreased muscle strength of the hemiplegic lower extremity, caused by immobility and the ensuing decreased daily functionality, was independently correlated with greater declines in BMD in early stage stroke patients, recognizing, again, that a considerable number of stroke patients may have experienced reduced BMD prior to the event. A greater risk of fracture poststroke can also be attributed to a high prevalence of prestroke low BMD and the occurrence of vertebral fractures, as previously described by Kim et al. .
Other analyses reinforce these findings. Prior to the Kim study, Watanabe used BMD measurements to record the presence of prestrike OP in 40% of 83 stroke patients (mean age=65.7 years), leading to the conclusion that greater attention should be paid to OP in subacute stroke patients . Subsequently, Kwan et al. confirmed both results in an analysis demonstrating a high prevalence of low BMD in 121 first-stroke patients (48 male, mean age=71.11±8.90; 73 female, mean age=68.61±8.42). In the male patients, 22.9% were osteoporotic; in the female the percentage was 78.1%, pointing to the high risk of fractures and the need for active intervention from the acute stages of stroke .
41.3.2.2
Immobilization, reduced physical activity, bone load reduction
Rapid bone resorption following stroke can be attributed, in large measure, to immobility, the resulting reduction in physical activity, and regional bone loss in, for example, the hemiplegic hip and upper limb . The primary underlying factors of low bone mass include the degree of paresis, the extent of generalized weakness (hemiparesis), duration of immobilization, severity of functional deficits, and time and extent of functional recovery. Whereas fractures cause a compensatory upregulation of osteoblastic activity, immobility and bone unloading result in a decrease in osteoblastic activities leading to cortical thinning .
On the paretic side, 1-year BMD loss in patients who remained wheelchair bound compared to those who relearned to walk within the first 2 months and to those who were able to walk throughout the 12 months of the study were 13%, 8%, and 3%, respectively, indicating a significant trend with ambulatory level and suggesting a role for both mobility and weight-bearing as critical factors in bone loss . In addition, during an immobilization period of 3–32 months, BMD loss in the total hip or femoral neck accelerated when the duration of hemiplegia is prolonged . In terms of the interaction between reduced weight-bearing and low BMD, stroke patients bearing less weight on the paretic leg (<50%) had faster bone loss and compromised bone density in the femoral neck than did those bearing more weight (>50%), indicating that reduced weight-bearing is a predictor of bone loss in stroke .
41.3.2.3
Muscle weakness, spasticity, and chronic disuse
It is generally recognized that muscle weakness and spasticity occur in the upper extremities of stroke patients, resulting in disuse of the paralyzed arm, bone demineralization, and fractures. The prevalence of poststroke spasticity is highly variable, ranging from 4% to 27% over the first 1–4 weeks, 19%–26.7% over 1–3 months, and 17%–42% beyond 3 months . Poststroke falls are likely to occur more frequently with increasing spasticity .
Studies conducted by Pang et al. have shown a negative association among spasticity, bone mineral content, and BMD . Using the Modified Ashworth Scale (MAS), they determined that spasticity in itself accounted for 23.2% of the variance in cortical thickness between the paretic and nonparetic side in the upper extremity of stroke patients, a finding that is perhaps attributable to the fact that spasticity exerts a high impact on upper extremity function and muscle strength. Greater demineralization was also associated with high levels of spasticity. In a subsequent study the same authors found that the greater the spasticity in the distal tibia, the lower the BMD at the distal tibial epiphysis . At the same time, there was no statistically significant correlation between lower extremity spasticity and functional mobility variables (ambulation and transfer ability). This implies that reducing spasticity may not result in improved function due to concurrent impairments such as muscle weakness .
41.3.2.4
Falls and fractures
Stroke patients are known to be at a higher risk of fractures than are age- and sex-matched populations absent stroke. One of the first investigations of fractures after stroke, undertaken by Ramnemark et al. in 1998, showed that of 1139 stroke patients followed for a median of 2.9 years, 120 subjects experienced 154 fractures, with a median time between stroke onset and first fracture of 2 years . Women had significantly more fractures than men, and 84% of the fractures were caused by falls. Hip fractures were the most frequent and their incidence was —two to four times higher than that of an age-matched reference population. Twenty years later, a systematic review and Bayesian metaanalysis indicated that stroke was associated with an over twofold increased risk of hip fractures (relative risk (RR)=2.11, 95%, CI 1.62–2.75) . Indeed, some estimates of risk of fractures point to as high as a fourfold increase.
However, important new research has focused on how much of the fracture risk is due to stroke and how much to other factors such as age and comorbidity . A large observational study, conducted by the Ontario (Canada) Stroke Registry in 2017, compared 23,750 hospitalized stroke patients with 11,240 patients hospitalized with transient ischemic attack (TIA)—a condition similar to stroke, but without the residual neurological impairment that is thought to be the principal mechanism accounting for increased fractures in stroke. Stroke subjects had a 32% increased risk of low-trauma fractures compared with the TIA group, and a 47% increased risk compared with age-/sex-matched controls at 2 years poststroke. In comparison with patients who experience a mild or severe stroke, patients with a moderate stroke are at the greatest risk of falls; their limited mobility causes a loss in BMD while, at the same time, accentuating their tendency to fall.
This study is differentiated from others in that the comparison between stroke and TIA enabled researchers to quantify the fracture risk most directly attributable to stroke-related neurologic events as opposed to other confounders, including, in descending order , older age, female sex, moderate stroke severity, prior falls and fractures, preexisting OP, atrial fibrillation, rheumatoid arthritis, and hyperparathyroidism—conditions that are linked with OP and fractures in the broader population. The association of demographic, stroke-related, and comorbid factors with fractures in stroke was confirmed in a subsequent study by Foster et al. that gave added emphasis to chronic kidney disease as a contributing factor for falls in the first year after stroke due to loss of bone and muscle mass. Both analyses assert that poststroke, greater attention should be focused on these factors, in addition to neurologic deficits, in an effort to identify patients at the greatest risk of falls and fractures.
41.3.2.5
Vitamin D and other nutritional deficiencies
Poststroke inhibition of PTH secretion, reduced sunlight exposure, and low intake of food rich in vitamins D, K, and B, coupled with suboptimal nutritional status, may all be contributing factors for OP. Hypercalcemia attributable to bone unloading blocks and/or reduces PTH secretion, thus preventing the renal synthesis of vitamin D 1,25(OH). There are various interpretations of how and to what extent lack of vitamin D is implicit in OP following stroke. Whereas a British study by Uluduz et al. found vitamin D deficiency and OP to be highly prevalent in stroke survivors, it found no direct association between the two entitles, after adjusting for potential confounders . However, there remains the indisputable fact that vitamin D is required to absorb the calcium needed to increase and ensure bone density and strength, help alleviate fractures, and reduce the risk of OP.
Immobilization in stroke patients reduces sunlight exposure, increasing the likelihood of reduced vitamin D. It may also cause hyperparathyroidism and accelerated bone remodeling, resulting in reduced PTH secretion, which further inhibits renal synthesis of vitamin D 25(OH) . The precise role of elevated PTH in this axis has yet to be determined. In a large ( n =15,792 men and women), biracial, and population-based 2014 study of PTH concentration and cardiovascular disease, Folsom et al. emphasize that PTH and vitamin D have an inverse relationship: when vitamin D is low, PTH is generally high, indicating that elevated PTH can be a marker for vitamin D deficiency . Moreover, the principal finding of their research, namely, the absence of any positive association between PTH levels and the risk for any cardiovascular diseases, including stroke, appears to indicate that vitamin D levels influence cardiovascular health to a greater degree than does PTH.
To the extent that generalized studies concerning the relationship of vitamin K to bone can be applied to stroke, two analyses, in particular, indicate an association between deficient vitamin K and hip fractures. Together with vitamin D deficiency, low levels of vitamin K are thought to be the most likely cause of subnormal carboxylation of osteocalcin (OC), a bone turnover marker linked with bone formation. The correlation between undercarboxylated OC (ucOC) and a hip fracture risk has been shown to be six times higher in elderly women with abnormally high values of serum ucOC . A subsequent study of hip fracture in an elderly population found vitamin K1 and vitamin D 25(OH) to be lower in fracture patients than in controls, with both being independently and synergistically associated with fracture risk . Poststroke subjects also evidence folate, B6, and B12 deficiencies that are associated with elevated Hcy levels and ultimately an increased fracture risk. Although further research is needed on the plasma concentrations of vitamins D, K, and B in stroke, there is sufficient evidence to warrant monitoring of these nutrients early in the poststroke period .
41.3.2.6
Pharmacological treatments for poststroke conditions
Aspirin, anticoagulants, and antiepileptic drugs (AEDs) are commonly administered to patients poststroke. According to the most recent American Heart Association/American Stroke Association stroke management guidelines , aspirin is still recommended for acute stroke patients within 24–48 hours of stroke onset, but urgent anticoagulation (such as intravenous heparin) is no longer indicated for most stroke patients. For many years, unfractionated heparin was routinely employed in the longer term to prevent and treat a potential venous thromboembolism poststroke, but its use has been largely discontinued with the development of better alternatives. Those patients who did receive unfractionated heparin in earlier years experienced its known inhibition of osteoblast differentiation and function, leading to decreased bone formation .
Questions have also been raised about the continued use of sodium warfarin, one of the oldest anticoagulant drugs and generally regarded as a vitamin K antagonist. A number of small studies, not specific to stroke, indicate that warfarin may be associated with lower BMD as well as increased vertebral and rib fractures . Yet, other analyses have found no significant effects on BMD or fractures in warfarin patients compared with controls .
In addition to its potential negative effect on bone, a number of other shortcomings in warfarin use, including the need for regular monitoring and increased bleeding risk, have led to the development of nonvitamin K oral anticoagulants (NOACs) such as the factor Xa inhibitors, apixaban, edoxaban, and rivaroxaban, and the thrombin inhibitor, dabigatran. These newer medications provide a clinical net benefit compared with warfarin and are recommended by international clinical guidelines. A recent metaanalysis of 12 randomized controlled trials (RCTs), comparing 44,816 patients on NOACs with 44,733 patients on warfarin, showed that the use of NOACs is associated with a significantly lower risk of fractures compared with warfarin, but with a relatively low absolute risk reduction . The difference may be attributable to the fact that warfarin exerts an adverse effect on vitamin K and imposes a dietary restriction on vitamin K intake, whereas NOACs tend to increase bone volume, lower bone turnover, and marginally increase BMD with little effect on vitamin K. However, further research is needed to determine the relative efficacy and safety of the two drug classes.
Both types of AEDs, enzyme inducers (phenytoin, phenobarbital, carbamazepine, and primidone), and enzyme-inhibitors (valproic acid), lead to accelerated bone loss and increased risk of fractures . The adverse effect of AEDs on bone is a major concern given the fact that treatment is administered for an extended period or even for a lifetime. An overall summary of causes of OP in stroke appears in Table 41.2 .
| Physical factors | Preexisting conditions | Anticoagulant medications |
|---|---|---|
| Weakness preventing FWB or PWB | Postmenopausal osteoporosis (females) | Unfractionated heparin for DVT prophylaxis |
| Spasticity and contractures preventing FWB on hemiplegic limb | Established senile osteoporosis (older males) | VKOR-inhibitors (e.g., warfarin) |
| History of low vitamin D | Direct oral anticoagulants (e.g., apixaban and rivaroxaban) a | |
| Fear of falling prompting decreased attempts to do even PWB | ||
| Family reluctance/fear of injury to continue PWB/FWB in home |
| GI prophylaxis medications | Antidepressant medications | Antiepileptic medications |
|---|---|---|
| Often added for GI protection in patients on anticoagulation | High incidence of poststroke depression; often added to enhance participation and facilitate rehabilitation | Added for short-term seizure prevention in those with large-territory or hemorrhagic stroke. Only certain agents are linked to alterations of calcium metabolism |
| Proton-pump inhibitors —block calcium resorption from the gut | ||
| SSRIs —associated with low BMD, increased falls, and greater nonspine fractures, including hip fractures | ||
| Phenytoin —accelerate vitamin D catabolism and adversely affects osteoblastic cells | ||
| H2-blockers —reduce calcium from the gut | ||
| Carbamazepine —similar effects as phenytoin | ||
| SNRIs —similar to abovementioned column but the noradrenaline component helps to mitigate negative effects | ||
| Phenobarbital —less commonly used, similar concerns as abovementioned agents |
a Unknown effect, but likely lesser negative effect on BMD compared to other anticoagulants.
41.3.3
Management strategies
As of this writing, poststroke OP is not specifically considered in the United States and other national guidelines for OP diagnosis and management, nor do guidelines for hip fracture prevention and management refer to poststroke bone loss. Nonetheless, the existence of a residual walking deficit is widely recognized as a critical factor in assessing the risk of OP in the lower limbs of a stroke patient. In the absence of a walking deficit, the presence of other risk factors for OP still dictates the need for a BMD screening to determine baseline values of bone density and to identify stroke patients who may already have OP or will soon require OP treatment . A manual muscle test to evaluate the function and strength of hip and knee extensors, the MAS to measure spasticity, and the Modified Barthel Index of activities of daily living to assess daily functionality, as well as other balance and mobility tests, may be performed as warranted.
41.3.4
Treatment options
41.3.4.1
Nonpharmacological interventions
Exercise
Experts agree that the single most important nonpharmacological treatment for OP in stroke is physical activity initiated early after stroke onset. Early results are promising. In 2005 Pang et al. issued a report on a 19-week Fitness and Mobility program employing a series of weight-bearing exercises reporting gains not only in cardiorespiratory fitness, mobility, and paretic leg muscle strength but also in maintenance of femoral neck BMD on the paretic side, as opposed to a 2.5% decline in the controls .
The following year, Pang used peripheral quantitative computed tomography to determine the effect of a similar 19-week exercise program on bone geometry more than 1-year post chronic stroke . Compared with controls, patients who completed skeletal loading, aerobic strengthening, and balance exercises had a significantly greater percent gain in trabecular bone content at the distal side of the tibia as well as a significantly greater gain in cortical thickness at the tibial shaft. However, no significant difference in bone density between the two groups was observed. Any effects on total hip or femoral neck BMD reported from this study? I find none . The potential of community-based group exercise programs is clear, but continuation of exercise after the termination of the program is essential and optimal timing for specific exercises (i.e., daily weight training for a minimum of 60 minutes for males and 90 minutes for females) is needed to stimulate significant BMD increases at lumbar spine and femoral neck , as demonstrated in a 2017 study by Han et al. Additional high-quality studies of over 2-year duration, with larger sample sizes and consistent outcome measurement, are needed to determine whether skeletal loading exercise and mobilization, coupled with avoidance of long periods of bed rest, are effective in reducing bone loss and fracture risk .
In view of the rapid and pronounced bone loss poststroke, the effect of early physiotherapy is receiving increased attention. Research shows that recovery of walking ability and higher motor function achieved within the first 6 months of a moderately severe stroke are associated with less bone loss over the period . Given the patients’ average age of 70, these results may not be applicable to older subjects, but the findings warrant further investigation. In a related study, researchers demonstrated that arm cycling training activated interlimb networks that aid in the coordination of rhythmic walking, months and even years poststroke .
Although exercise may improve functional status and mobility in stroke patients, it does not appear to have a protective effect against fractures; in fact, greater mobility may increase the likelihood of fractures, especially as patients return home. A recent Taiwanese study found that patients undergoing rehabilitation have higher incidence of vertebral, pelvic, femoral neck, and humeral fractures than those without rehabilitation, but a significantly increased fracture risk has been observed only in older women. Assistive hip protectors, seizure pads on the ground at bedside, and other environmental modifications may assist in fall and fracture prevention.
Dietary supplementation
It is generally agreed that calcium and vitamin D supplementation should be instituted for stroke survivors on a case-by-case basis to ensure that they are calcium and vitamin D replete. Although an association between low vitamin D level and loss of BMD in general is subject to varied interpretations, a recent investigation on stroke patients showed no correlation between vitamin D level and bone density in a relatively small sample . It did, however, indicate that the risk of vitamin D deficiency increases to a greater extent for patients in a prolonged nonambulatory state as they experience hypercalcemia and the resulting inhibition of PTH secretion. At present, the efficacy of vitamin D and calcium supplementation in patients after stroke awaits further review in larger, adequately powered clinical trials that take into account dosing, timing, and sunlight exposure.
41.3.4.2
Pharmacologic interventions
Oral and intravenous bisphosphonates
In addition to the efficacy of exercise and the potential of vitamin D in restoring and maintaining bone mass following stroke, bisphosphonates are now regarded as a viable therapy for OP in stroke. Given the lack of studies directly relevant to stroke, use of oral bisphosphonates poststroke must be based on generalized studies regarding their efficacy in attenuating bone resorption while taking into account the need to remain upright for 30 minutes prior to drug administration—an impossibility for many stroke patients—and the tendency toward noncompliance, a serious concern in stroke patients, particularly in those with dysphagia and reflux. Relatively rare adverse effects of bisphosphonates must also be considered in all patients .
Potent intravenous bisphosphonates obviate such concerns. An randomized controlled trial (RCT) focusing on the effect of ZA on bone loss in patients with acute stroke demonstrated that a single intravenous dose (4 mg) administered within 5 weeks of hospitalization effectively prevented bone loss in the hemiplegic hip for at least 1 year after administration . Before initiating intravenous bisphosphonate treatment, patients should undergo regular assessment of renal function and hydration. Again, larger, high-powered studies are needed to provide further information about the use of oral and intravenous bisphosphonates in stroke. There are no studies evaluating ability of bisphosphonate medication to reduce risk of hip fracture in stroke patients.
Statins
In recent years an exciting new prospect for treating poststroke OP has emerged in the form of statin use. For some time, it has been known that statins taken before and during stroke hospitalization are associated with improved poststroke survival, while statin withdrawal during hospitalization substantially lowers the survival rate . Now, there is new evidence that statin use may also decrease OP and fracture risks in stroke patients. In a population-based study of newly diagnosed stroke patients without a history of OP , Lin et al. reported a decreased risk for OP as well as for hip and vertebral fractures in 2627 patients treated with statins, as opposed to the same number of nonstatin users. Subanalyses focusing on a dose–effect relationship found that only patients with high cumulative daily doses of statin demonstrated a significant decreased risk.
Hundreds of thousands of people throughout the world experience long-term disability and severe comorbidities, including OP, following stroke. In this context, physicians must be made aware of the prevalence of OP poststroke and the need to provide appropriate treatment. An important first step would be the inclusion of OP assessment and treatment in national and international stroke-specific guidelines. As indicated previously, the importance of differentiating between stroke-induced OP and other causal factors must be recognized. In terms of treatment options, current evidence emphasizes nonpharmacological treatment, particularly early mobilization, and intravenous ZA, as the most prudent and effective approach, coupled with continued investigations of all forms of early intervention in the face of rapid and severe bone loss poststroke.
41.4
Multiple sclerosis
MS is an inflammatory, autoimmune, neurodegenerative disease of the central nervous system (CNS) that damages the myelin sheath of nerve fibers, leaving a scar or sclerosis and exposing nerve fibers that can then no longer transmit messages from the brain to muscles. Affecting the brain, spinal cord, and optic nerves, the symptoms of MS emerge over the course of a few days or weeks and include numbness in the limbs, muscle spasms, double-vision and hearing loss, seizures, and paralysis. Like Parkinson’s, MS patients evidence trembling hands, spastic limb movements, and poor balance; unlike both Parkinson’s and stroke, MS presents in younger individuals, generally between the age of 20 and 50. In some cases the inflammation evident in MS can result in brain lesions that, in turn, lead to Parkinson’s .
41.4.1
Epidemiology and risk factors of multiple sclerosis
In 2019 the National Multiple Sclerosis Society issued confirmed findings of a prevalence study, estimating that nearly 1 million people in the United States are now living with MS, nearly twice the number of 400,000 reported more than 40 years ago . Although it did not break down the prevalence of MS by specific types, it did report that women now make up 74% of MS patients in the United States as opposed to 63% on 1975. It also confirmed the correlation of MS prevalence with distance from the equator, reporting an MS prevalence of 377 per 100,000 for people living in the nine Northeastern states as compared with a 277 per 100,000 for those in the South and West.
By contrast, 2.3 million people live with MS worldwide. The highest prevalence was in high-income North American, Western Europe, and Australasia as expected, given their distance from the equator and corresponding lack of sunlight; the lowest was in eastern and central sub-Saharan Africa and Oceania .
Although the cause of MS is unknown, it occurs, in the most immediate sense, when an abnormal immune response leads to inflammation and damage to the CNS (as indicated previously). The interaction of activated T cells and B cells, entering the CNS through blood vessels, destroy the substance that covers nerve cells, known as myelin, thereby damaging the nerves’ ability to conduct impulses between the brain and other parts of the body . What precipitates and underlies this abnormality is subject to varied interpretations, involving genetic susceptibility and environmental determinants, including infections. In a model developed by Goodin , genetic susceptibility, specifically family history of autoimmune disease, emerges as the most important determinant of MS given the fact that at least 92.5% of individuals are incapable of developing MS, regardless of specific environmental exposures. Given this genetic makeup, the environmental factors that trigger the abnormal immune response can range from the geographic gradient and the low vitamin D levels cited previously to smoking, obesity, and chronic infections, particularly the Epstein–Barr virus. Although men are likely to be more genetically susceptible to MS, the disease is more common in women largely because they are more responsive to environmental exposures. Another potential risk factor for MS, head trauma in adolescence, has also recently been identified .
41.4.2
Types of multiple sclerosis
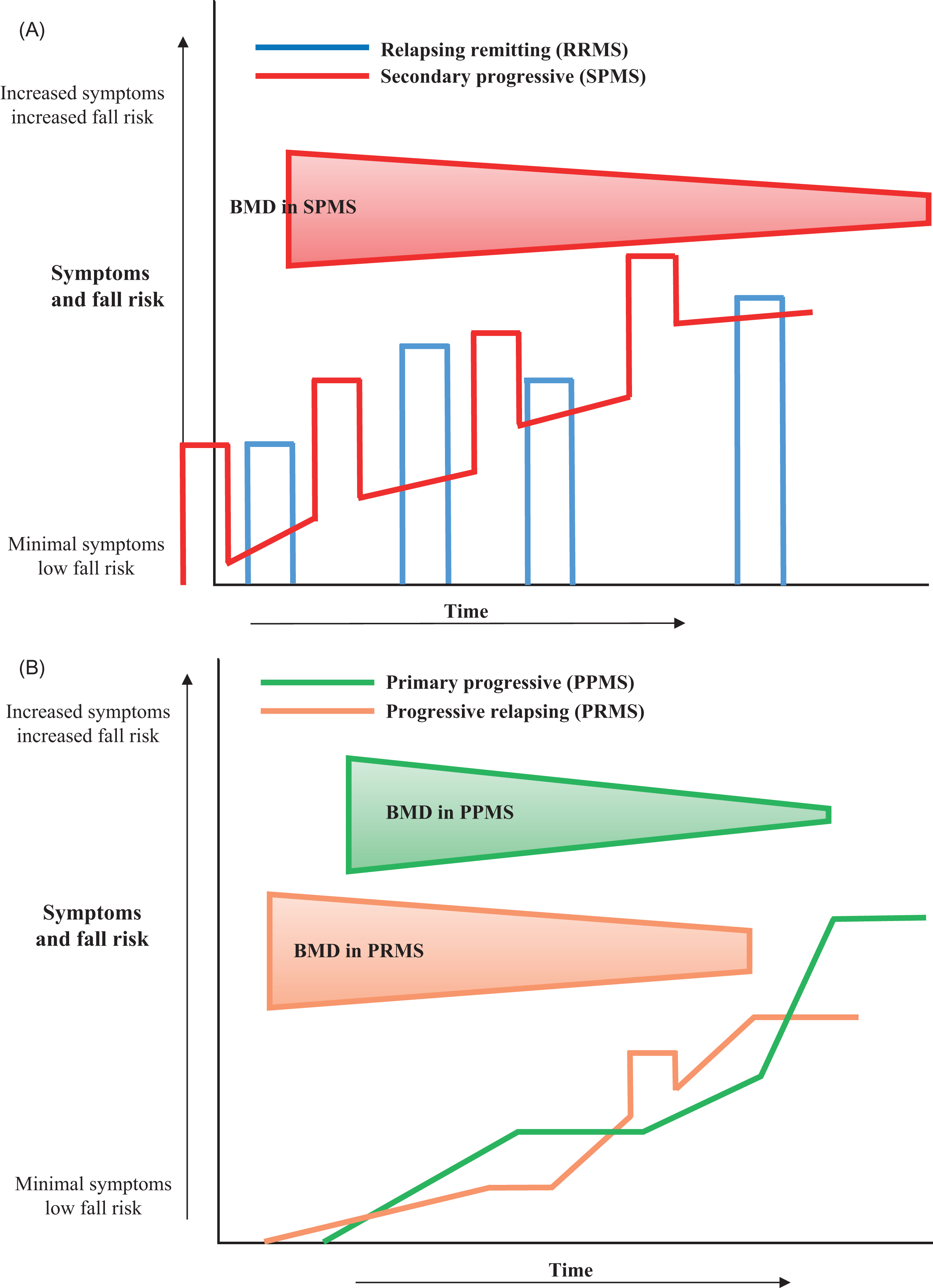
The four different types of MS are distinguished by their impact on the body, generally indicating how the disease will progress over time . The most prevalent, affecting primarily females, are relapsing-remitting MS (RRMS) and secondary progressive MS (SPMS), which together constitute 85% of MS cases. RRMS is characterized by attacks of new or increasing neurological symptoms: the relapses, flare-ups, or exacerbations, followed by a return to either baseline functioning or partial recovery—the remissions. SPMS begins in a manner similar to RRMS but transitions to a steady progression of worsening symptoms over time, with or without relapses and remissions. The two less prevalent types, accounting for 15% of cases, are primary progressive MS in which patients continue to decline without relapses or remissions and the rare progressive-relapsing MS in which the disease steadily worsens from the onset with relapses but no remissions .
Studies indicate that progressive forms of MS result in greater bone loss than do relapsing-remitting forms, given differences in functional status as demonstrated in the Kurtzke Expanded Disability Scale (EDSS). Fig. 41.2 illustrates the progression of symptoms and fall risk in respective types of MS.