37.1
Introduction
Childhood and adolescence are critical periods for bone mass acquisition. Through skeletal growth, the peak of bone mineral accrual occurs approximately 6 months following the pubertal growth spurt, and increased bone density continues throughout adolescent and young adult years . Genetic programing, epigenetic modifications, physical activity, hormones, and nutrition play an important role in bone mass determination. Interruption in the interplay of these factors could potentially lead to compromised gains in bone mineral or even bone loss. Although many of primary and secondary skeletal disorders in children can also afflict adults, their presentation in childhood is often unique. There are also disorders that uniquely affect children and adolescents, especially genetic diagnoses. These disorders tend to affect acquisition of optimal bone size, cortical thickness, and bone mass in children and adolescents. Therefore the therapeutic goal, unlike in adults, is not only aimed to halt bone loss but also to foster skeletal gains.
Although fractures are common in adolescents, as one-half of boys and 40% of girls have sustained at least one fracture before the age of 18 years, low-trauma fractures of the vertebrae and lower extremity bones in children should prompt suspicion for bone fragility secondary to genetic causes or acquired chronic disorders . While the World Health Organization defines osteoporosis in adults as a bone mineral density (BMD) 2.5 standard deviations below peak ( T -score <2.5), the diagnosis of pediatric osteoporosis cannot be made based on bone densitometric criteria alone as population-based data on correlation between BMD and fracture rates and risk in healthy children are not available . The 2013 Pediatric Position Development Conference of the International Society of Clinical Densitometry (ISCD) developed the official recommendations on use of bone densitometry and diagnosis of osteoporosis in childhood . One of the key conclusions was that fragility fractures of the long bones or vertebral compression fractures are needed to establish the diagnosis of pediatric osteoporosis.
While the treatment of osteoporosis is first focused on addressing risk factors associated with low BMD, pharmacologic therapy may be needed in children with primary osteoporosis or in those with secondary osteoporosis whose risk factors are unlikely to be mitigated. Anti-resorptive agents (e.g., bisphosphonates) have long been used for the treatment of pediatric osteoporosis on a compassionate care basis, primarily in osteogenesis imperfecta (OI), a hallmark disease of pediatric osteoporosis. Experience and data on the safety and efficacy are sparse in other forms of pediatric osteoporosis, and even less is known about use of anabolic agents in children.
The purpose of this chapter is to summarize current data on the diagnosis and management of pediatric osteoporosis and other disorders associated with bone fragility in childhood and adolescence. Detailed information regarding the principle of bone mass acquisition can be found in Chapter 36 , Bone mineral acquisition in utero and during infancy and childhood.
37.2
Definition and diagnosis of osteoporosis in children and adolescents
37.2.1
Definition
Osteoporosis is a skeletal disorder characterized by compromised bone strength, low bone mass, or an alteration in bone microarchitecture predisposing to an increased risk of fracture . The relationship between BMD and fracture risk is well established in population-based studies in adults . A BMD T -score less than −2.5 defines osteoporosis, and T -score between −1.0 and −2.5 is consistent with osteopenia . Because a large percentage of fractures occur when T -score is above −2.5, clinical risk factors that are independent of BMD, such as older age, prior history of fracture, low body mass index (BMI) glucocorticoid use, alcohol consumption, and cigarette smoking, are also important predictors of fracture risk . While BMD is an essential tool to identify adults who are at risk before fractures occur, the association between a BMD threshold and fracture risk in children is not well established. The diagnosis of osteoporosis in children should therefore not be made on the basis of low BMD alone . The 2013 ISCD Official Positions Statement suggest that the diagnosis of osteoporosis be restricted to children and adolescents with (1) one or more vertebral compression fractures in the absence of high impact trauma or local disease or (2) clinically significant fracture and BMD Z -score less than −2.0 . A clinically significant fracture is defined as 2 or more long bone fractures by age 10 years, or 3 or more long bone fractures at any age up to 19 years. ISCD also suggests that normal BMD does not preclude the diagnosis of osteoporosis or increased fracture risk.
37.2.2
Bone densitometry
Bone densitometry is an invaluable tool for a comprehensive bone health assessment in children and adolescents with a concern for bone fragility. This section highlights challenges in performing and interpreting bone densitometry and its utility in predicting fracture and osteoporosis in growing children. Densitometric techniques used to assess bone health are discussed in detail in Chapter 64, Noninvasive imaging techniques and fracture risk assessment.
37.2.2.1
Dual X-ray absorptiometry
Dual X-ray absorptiometry (DXA) is the most widely used densitometry techniques in children because of its speed, reproducibility, availability, less radiation exposure, and robust age-appropriate reference data . DXA has remained the preferred method for assessment of bone mineral content (BMC, g) and areal BMD (aBMD, g/cm 2 ) . Anterior–posterior lumbar spine (LS) and total body less head (TBLH) are the recommended site of scans because the bony landmarks allow for better reproducibility in growing children. LS (L1–L4) DXA provides information on the status of trabecular bone as this region of the spine is a trabecular-rich site. Reference ranges for the LS DXA are available in children 1 month to 20 years of age . The skull is ideally excluded from total body analysis because the mineral content in this region comprises as much as 20%–40% of total body mineral content and is usually preserved with changes in nutritional or environmental factors that affect bone mineral . TBLH DXA provides not only information on the status of cortical bone but also body composition that may guide clinicians while evaluating children with chronic diseases. Normative data of TBLH are available for children age 5–20 years .
While total hip and proximal femur are recommended sites for DXA scans in adults, they have limited utilization in younger children because of difficulties in positioning and identifying bony landmark, leading to lower reproducibility. In clinical practice, total hip DXA can a useful tool for BMD assessment during mid-to-late adolescence years as skeletal landmarks are close to full development . Normative data for total hip DXA are available for children age 6–19 years . Distal radius and lateral distal femur (LDF) comprise primarily cortical bone and are potential assessment sites for children and adolescents who cannot undergo LS or TBLH scans, particularly children with severe contracture or indwelling metal hardware. The association between low BMD of the distal radius and forearm fracture has previously been demonstrated in children, and normative data are now available for this skeletal site , although with lower reproducibility than other sites . Low BMD at LDF has been shown to be associated with an increased risk of fractures in children with impaired mobility . At the present time, LDF analysis can only be generated using the distal radius DXA software and semistandardized analysis techniques. Reference ranges for LDF DXA are available in children age 3–18 years .
Misinterpretation of densitometry in children is common and often leads to unnecessary additional testing and treatment . One of the most common pitfalls is using T -score to make the diagnosis of osteoporosis in children. An appropriate interpretation of DXA value in children and adolescents requires a standard deviation or Z -score of BMC and aBMD, as compared with healthy children at same age and sex, using manufacture-specific pediatric reference data. Since aBMD and BMC are obtained by an areal measurement, they are not true volumetric density and are largely influenced by bone size—falsely low in shorter individuals and falsely elevated in taller individuals. To account for bone size, LS and TBLH BMC and aBMD Z -scores should be adjusted for absolute height . LS BMD can also be adjusted using bone mineral apparent density (BMAD) . Delay of bone growth and maturation that often accompany chronic disease in children also affects DXA interpretation. Height age, bone age, and body composition adjustments have also been proposed to reduce the number of children inappropriately identified as having low bone mass , although none of these methods has been validated as a predictor of future fractures .
DXA is the only recommended densitometric method for clinical assessment of fracture in otherwise healthy children . Although children who sustained fracture following forearm injury had lower aBMD when compared with peers who did not fracture , the predictive value of both BMC and aBMD in future fractures in children is still limited. Data from few studies have shown that low BMC and aBMD at LS and TBLH were associated with increased risk for upper extremities fractures . Whether low bone density in childhood and adolescence increases the risk of fractures in adulthood has yet to be elucidated.
The limitations of clinical utility of DXA in children merit considerations. As aforementioned, DXA provides areal rather than volumetric assessment of bone mass and greatly influenced by bone size. Although information about cortical and trabecular bone compartment can be indirectly extrapolated from scan sites, DXA does not provide direct measurements of these skeletal compartments. Lastly, the quality of bone is not determined only by bone density but also bone geometry and properties of the organic and mineralized matrices. Alternative densitometric techniques have been developed to understand definitively skeletal development and skeletal changes in children, although are still limited to the research setting.
37.2.2.2
Trabecular bone score
By utilizing the standard LS DXA, trabecular bone score (TBS) measures the variation of gray level to compute an index of trabecular bone microarchitecture of the spine. A higher TBS indicates stronger skeletal microarchitecture. The ability of TBS in predicting fracture risk independently of BMD by DXA is well demonstrated in adults . To date, there have been only a few studies utilizing TBS for bone health assessment in children and adolescents . Data from a study of 338 healthy adolescents have identified significant correlations between TBS and age, pubertal stages, body composition, and spine and total aBMD and BMC . A cross-sectional study of 57 girls with anorexia nervosa has shown correlation between LS TBS and aBMD by DXA, body composition, and stress–strain index obtained by peripheral qualitative computed tomography (pQCT), but not fracture history . Another longitudinal study utilized TBS for monitoring of anti-resorptive therapy in eight children with OI . There was a greater change in aBMD when compared with TBS in denosumab-treated group, suggesting greater effect of denosumab on cortical bone. Presently, TBS is still limited in research given the lacks of normative data in children and powered study to examine the prognostic value of TBS on fracture risk in children.
37.2.2.3
Peripheral qualitative computed tomography
pQCT provides three-dimensional measurements of the volumetric BMD (vBMD) that is unaffected by bone size. pQCT also measures bone geometry and distinguishes cortical from trabecular compartments, thus rendering the examination of bone distribution at fracture-prone sites. The 3% or 4% distal tibia or radius are commonly used to assess the trabecular compartment, while cortical BMD can be measured at the 38% distal tibia, or the 20% or 50% distal radius . Because cortical and trabecular bone responses to pubertal changes, mechanical forces, and chronic disease may differ, pQCT holds promise for more specific classification of abnormalities of bone mineral acquisition during childhood. The clinical application of pQCT use for bone health assessment in children has been demonstrated in healthy children and young adults . In a cross-sectional study of healthy children age 5–16 years, children who sustained forearm fractures had lower vBMD, cortical area, and strength–strain index of the distal radius when compared with children who sustained forearm injury but did not fracture .
While pQCT measures allow for the evaluation of bone structure and bone strength indices, such as cortical vBMD, cortical cross-sectional area, periosteal and endosteal circumferences, and polar strength–strain index, the resolution of pQCT is not sufficient to quantify bone microarchitecture. High-resolution pQCT (HRpQCT) provides a more refined characterization of bone microarchitecture. Studies in adults have demonstrated the ability of HRpQCT to assess fracture risk, independent of DXA . Findings generated from HRpQCT in children have shown the peak of cortical porosity to occur during mid-puberty , coinciding with the peak incidence of forearm fractures in children and young adolescents . To date, only a limited number of studies using HRpQCT for fracture assessment have been carried out in children .
Although the development of pQCT and HRpQCT has afforded more insight into bone microarchitecture and fracture risk and reference data for pQCT- and HRpQCT-derived measures of bone structure and strength in children are available , standardized protocols for performing these measurements are lacking. Furthermore, ensuring longitudinal measurements at the same region in growing children can be challenging. At present, pQCT and HRpQCT remain largely research tools.
37.2.3
Vertebral fracture assessment
Vertebral fractures, in the absence of high impact injury, are indicative of bone fragility irrespective of BMD. However, vertebral fractures have remained underrecognized as manifestation of poor bone health in children with chronic diseases as they are often asymptomatic .
Lateral spine radiographs may serve as useful evaluations to screen for vertebral fracture in children with chronic disease at risk for osteoporosis. Vertebral fracture analysis (VFA) by DXA software exposes children to less radiation exposure when compared with lateral spine radiograph. The diagnostic accuracy of VFA is comparable to lateral spine radiograph in adults with osteoporosis . VFA is not commonly used in clinical setting as less data are available in children and adolescents, although newer VFA software has shown sufficient image quality with adequate accuracy in identifying vertebral fracture in children .
The Genant semiquantitative method is widely used to characterize vertebral fractures in adults as well as children and adolescents . Using this method, vertebral bodies are graded based on difference in height ratios comparing the anterior, middle, and posterior vertebral heights to the heights of adjacent vertebral bodies ( Fig. 37.1A ). The differences in heights are scored as following: grade 0: 20% or less (normal), grade 1 fracture: greater than 20%–25%, grade 2 fracture: greater than 25%–40%, and grade 3 fracture: greater than 40%. Data from healthy children suggested that 95% of children across all age groups had no more than an 11% reduction in the anterior to the posterior vertebral height ratio . Algorithm-based qualitative (ABQ) method provides further guidelines to differentiate vertebral fractures from nonfracture deformity by evaluating endplate discontinuity. However, ABQ approach has not been widely used in pediatric population due to large physiological variations of shapes and end plates of growing vertebrae in children and adolescents .
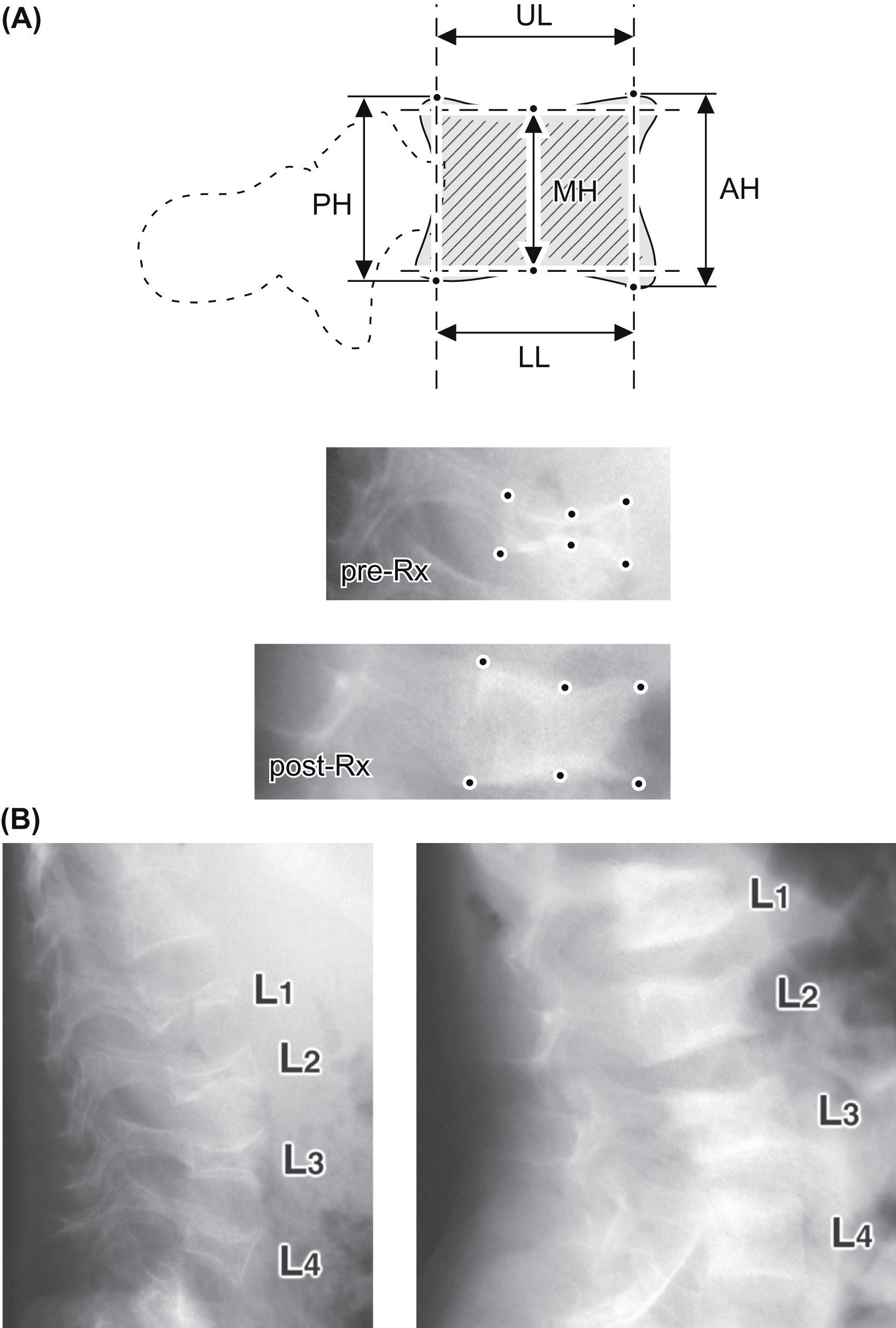
Data from large prospective, longitudinal studies of children with leukemia treated with systemic glucocorticoids have demonstrated the association between mild vertebral fracture (grade 1) and low LS aBMD Z -scores , and the presence of vertebral fracture at leukemia diagnosis was associated with increased risk of further fractures . Early identification of vertebral fractures is not only critical for identifying bone fragility but also guiding therapy since, unlike in adults, normalization of vertebral deformity or vertebral body reshaping is evident in children . Changes in the vertebral heights of lumbar vertebrae determined from lateral radiographs can be charted overtime to monitor progression of bone disease or the efficacy of treatment ( Fig. 37.1B ).
37.2.4
Bone turnover markers
There has been increasing use of bone turnover markers (BTM) for bone health assessment in children and adolescents with the availability of age-specific reference values . BTM can serve as an additional tool in making diagnosis of osteoporosis in children and teenagers. For instance, bone formation markers are generally low in osteoporosis pseudoglioma syndrome (OPPG) due to an LRP5 mutation. BTM has also been proposed as a monitoring tool for the efficacy and compliance of anti-resorptive therapy of osteoporosis . However, unlike in adults, caution is needed when interpreting BTM in children as there is high inter- and intraindividual variability of BTM in growing children due to many confounding factors such as age, pubertal status, growth rate, nutritional status, diet, and physical activity. In addition, BTM has not been correlated with change in aBMD or bone health outcome . Currently, there is insufficient evidence on the utility of BTM in predicting fracture risk in children, and the use of these markers in children and adolescents is mostly limited to clinical research. The one exception is use of a bone resorption marker (e.g., serum C-telopeptides) to rule out a low turnover state before the initiation of a bisphosphonate.
37.2.5
Bone histomorphometry
Bone histomorphometry is a quantitative analysis of undecalcified bone tissues, typically taken from the iliac crest. The insight generated from bone histomorphometry provides the opportunity to understand cell function and metabolism, and distribution of bone tissue. In addition, unlike other indirect tools to assess bone health, histomorphometry is not affected by bone size and bone growth, which is relevant to pediatric population. Despite its distinct advantages and the availability of normative data in children , bone histomorphometry is not commonly used in children, partly because it is an invasive procedure, and requires specialized skills and equipment to process and analyze the samples.
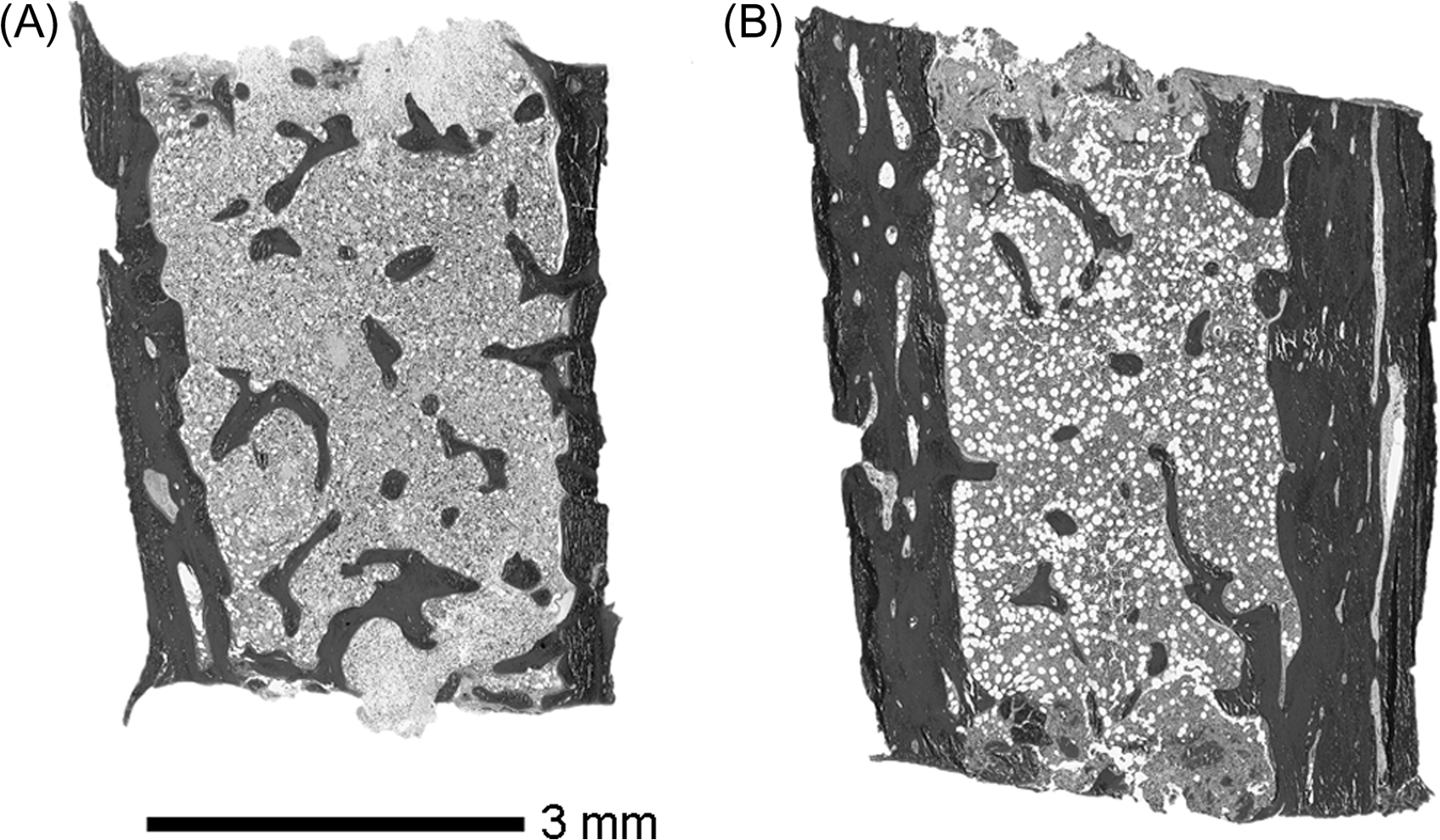
Bone histomorphometry is an invaluable tool when clinical history, radiography, BTM, and other biochemistry do not yield the etiology of the bone fragility. For example, idiopathic juvenile osteoporosis (IJO) is distinct from nonpathologic recurrent fractures in healthy children or mild forms of OI on the histological grounds—IJO usually has normal biopsy specimen size but with low bone turnover and bone cellularity, in contrast to OI . Histomorphometry can also delineate less common forms of OI with similar clinical history . Findings from clinical research utilizing histomorphometry have also been valuable in guiding pharmacologic therapy for osteoporosis in children. For instance, histomorphometric studies on children with OI have shown an increase in cortical thickness following pamidronate therapy, while trabecular thickness remained unaffected ( Fig. 37.2 ). In contrast, cortical thickness change was not observed in alendronate-treated children . These findings have led to diminished enthusiasm about the use of oral bisphosphonates as the first-line therapy in children with OI. Anabolic therapies, while not currently in use clinically, are under study.
In summary, while there have been advances in the development of techniques for assessment of pediatric bone health, the diagnosis of osteoporosis in children cannot rely on a single test, and the presence of overt bone fragility is still the key for making diagnosis.
37.2.6
Bone marrow adipose tissue assessment
Since the first observation of increased bone marrow adipose tissue assessment (BMAT) in osteoporotic bone , there has been growing evidence of the interactions between bone and BMAT . From postnatal period, BMAT gradually increases and replaces hematopoietic cells in marrow of the long bones. By age 25 years, over 50% of total marrow volume is occupied by BMAT . The development of BMAT is dynamic process in response to nutrition, environment, and hormones . In adults, BMAT assessed by newer magnetic resonance imaging and magnetic resonance spectroscopy techniques has been shown to inversely correlate with cortical bone area , BMD, and fracture risk . In children and adolescents, the inverse relationship between BMAT and BMD has also been observed . Premature increased BMAT has been reported in adolescent girls with anorexia nervosa . In contrary, adolescent boys with obesity were noted to have lower BMAT at L4 vertebra when compared with similar age adolescents with normal weight . To date, there are no data on the correlation of BMAT change and fractures outcome in children and adolescents. Further studies are needed as BMAT has potential to serve as a novel biomarker of pediatric bone health.
37.3
Etiology of osteoporosis in children and adolescents
The principles of skeletal development through infancy, childhood, and adolescence are reviewed in detail in Chapter 36 , Bone mineral acquisition in utero and during infancy and childhood. Childhood and adolescence years are critical period for skeletal development. Rapid increase of mineralization and bone size occurs during puberty. After final adult height is achieved, BMD continues to accrue and reaches the peak in the mid-to-late 20s. Bone mass itself does not fully address the skeleton’s functional role . Bone architecture and geometry are also essential components to maximize bone strength. While genetics plays a pivotal role in skeletal development during embryogenesis, postnatal mechanical challenges are also as important for the developing skeleton. Based on Frost’s mechanostat theory , Rauch and Schoenau proposed the functional model for bone development. In brief, when mechanical challenges exceed the bone mechanostat set point, bone formation develops in response to the need to withstand the challenge. Complex interactions between genetics, nutrition, physical activity, and hormones play an important role in bone mass development and the mechanostat model . Interruptions in these interplays could compromise bone health and eventually osteoporosis.
Disorders associated with osteoporosis in children and adolescents can be classified as primary or secondary ( Table 37.1 ). Primary osteoporosis is a pathological entity of primary bone diseases that are characterized by increased bone fragility, with OI, OPPG, and IJO being the hallmark diseases in children and adolescents . Other forms of connective tissue disorder such as Ehlers–Danlos syndrome, Marfan syndrome, and homocystinuria are also associated with low BMD and increased fracture risk. Other skeletal syndromes associated with significant bone fragility are polyostotic fibrous dysplasia, hypophosphatasia, and Cole–Carpenter syndrome.
|
Secondary osteoporosis due to a chronic disease or medications is usually a result of impaired bone mineralization (decreased bone formation or increased bone resorption), bone microarchitecture or bone size. While advances in medical care have improved survival outcomes in children with chronic diseases, many children, adolescents, and young adults may suffer from long-term complications, including osteoporosis, from their conditions or medical treatment. The diverse disorders associated with secondary osteoporosis typically share common risk factors, including chronic inflammation, impaired mobility, malnutrition, hormone disruption, and bone toxic medications. Frequently, not single but multiple risk factors contribute to the deterioration of bone health. For instance, progressive muscle weakness, chronic inflammation, delayed puberty, vitamin D deficiency, and chronic glucocorticoid treatment are all detrimental to bone mineralization and lead to refractory osteoporosis in children with Duchenne muscular dystrophy (DMD) . It is of utmost importance that clinicians identify these risk factors early and intervene to prevent the comorbidity associated with osteoporosis such as bone pain, fractures, and subsequent immobilization.
In this section, the pathophysiology, clinical manifestations, diagnosis and overview of the management of commonly reported disorders associated with osteoporosis in children will be discussed. Osteoporosis associated with eating disorders, another example of secondary osteoporosis, is discussed in Chapter 44, Osteoporosis associated with eating disorders.
37.3.1
Primary osteoporosis
37.3.1.1
Osteogenesis imperfecta
OI represents a group of heritable bone dysplasias characterized by generalized bone fragility, bone deformity, and low bone mass, with various phenotypes (see also Chapter 48: Osteogenesis imperfecta and other defects of bone development as occasional causes of adult osteoporosis). Once thought to be an autosomal-dominant disorder of collagen type I synthesis, the first gene responsible for recessive form of OI was identified in 2006 . Over the past decade the discovery of novel genes and advance in molecular bone biology have led to a better understanding of the genetics and mechanisms of OI. Prior to the identification of the collagen defect in OI, Sillence et al. classified OI into four types based on severity of clinical and radiographic findings . Nowadays, OI is appreciated to be a group of connective tissue disorders, ranging from defects in collagen synthesis or impaired posttranslational modifications to impaired bone mineralization, with more than 18 subtypes ( Table 37.2 ). Recently, changes in biomarkers of noncollagen type I have also been demonstrated in patients with OI, suggesting widespread of extracellular matrix alteration . The incidence of OI is estimated at 0.3–0.7 per 10,000 live births in North America and Europe .
| Osteogenesis imperfecta type | Mutated gene | Encoded protein | Inheritance | Bone phenotype |
|---|---|---|---|---|
| Impaired collagen synthesis and structure | ||||
| I | COL1A1 | Collagen α1 | AD | Mild |
| II | COL1A1 or COLA1A2 | Collagen α1 or collagen α2 | AD | Lethal |
| III | COL1A1 or COLA1A2 | Collagen α1 or collagen α2 | AD | Progressive deforming |
| IV | COL1A1 or COLA1A2 | Collagen α1 or collagen α2 | AD | Moderate |
| Impaired mineralization | ||||
| V | IFITM5 | BRIL (also known as IFM5) | AD | Variable |
| VI | SERPINF1 | PEDF | AR | Moderate to severe |
| Abnormal collagen posttranslational modification | ||||
| VII | CRTAP | CRTAP | AR | Severe to lethal |
| VIII | P3H1 / LEPRE1 | P3H1 | AR | Severe to lethal |
| IX | PPIB | PPlase B | AR | Moderate to lethal |
| Impaired collagen processing and cross-linking | ||||
| X | SERPINH1 | Serpin H1 (also known as HSP47) | AR | Severe to lethal |
| XI (Bruck syndrome type 1) | FKBP10 | 65 kDa FK506-binding protein (FKBP65) | AR | Progressive deforming and joint contractures |
| No type (Bruck syndrome type 2) | PLOD2 | LH2 | AR | Progressive joint contractures |
| XIII | BMP1 | BMP1 | AR | Mild to severe |
| Altered osteoblast differentiation and function | ||||
| XII | SP7 | Transcription factor SP7 (also known as osterix) | AR | Severe |
| XIV | TMEM38B | TRIC-B (also known as TM38B) | AR | Severe |
| XV | WNT1 | WNT1 | AR, AD | Severe |
| XVI | CREB3L1 | OASIS (also known as CR3L1) | AR | Severe |
| XVII | SPARC | SPARC (also known as osteonectin) | AR | Severe |
| XVIII | MBTPS2 | Membrane-bound transcription factor S2P | XR | Moderate to severe |
The classic forms of OI (types I–IV) as proposed by Sillence et al. are caused by autosomal-dominant mutations in COL1A1 or COL1A2 genes. Type I OI (mild) is a result of type I collagen haploinsufficiency from a COL1A1 mutation and is characterized by mild bone deformity, and near-normal stature. OI types II–IV are caused by mutations in COL1A1 or COL1A2 resulting in collagen type I structural abnormality. The severity of bone deformity in OI type II–IV ranges from moderate to perinatally lethal. Approximately 85%–90% of cases with OI are a result of quantitative or structural mutations in these collagen genes .
OI type V is caused by an autosomal-dominant IFITM5 mutation resulting in defects in bone mineralization . Distinct phenotypes of OI type V include intraosseous membrane ossifications and hyperplastic callus formation . Another form of OI also characterized by defects in bone formation and mineralization is OI type VI due to a mutation of SERPINF1 gene that encodes pigment epithelium–derived factor (PEDF) . PEDF is involved in osteoclast activity through receptor activator of nuclear factor-κβ ligand (RANKL) pathway, and the absence of PEDF due to null mutation in SERPINF1 leads to delayed mineralization . Patients with OI type VI have moderate-to-severe bone phenotype with lamellation pattern on bone histomorphometry .
Mutations resulting in defects in the posttranslational modification of collagen have also been recently identified as the causes of rarer types of OI with autosomal-recessive pattern of inheritance. Null mutations in CRTAP (OI type VII) and P3H1 (OI type VIII) lead to reduced hydroxylation of proline of the collagen α1 chain, thereby delaying its helix folding . These two forms of OI share indistinguishable bone phenotypes, including rhizomelia and severe to lethal osteochondrodystrophy . OI type IX is caused by mutation in PPIB and has a similar bone phenotype to type VII and VII, however, without rhizomelia .
OI types X, XI, and XIII are caused by defects in collagen processing due to mutations in collagen chaperones SERPINH1 , FKBP10 , and BMP1 , respectively. Clinically patients with OI type X have severe bone phenotypes with or without dentinogenesis imperfecta and hearing loss . Mutations in FKBP10 results in overlapping clinical manifestations of severe OI (type XI) and Bruck syndrome (severe OI with congenital joint contractures) . Type 2 Bruck syndrome has been reported to be associated with PLOD2 mutations . OI type XIII due to BMP1 mutation is characterized by mild-to-severe bone phenotypes .
The last group of rarer OI subtypes (types XII and XIV–XVIII) is caused by defects in osteoblast differentiation or bone mineralization, with or without collagen pathway being affected. Mutations in SP7 , TMEM38B , WNT1 , CREB3L1 , SPARC , and MBTPS2 have been identified in moderate-to-severe types of IO with various patterns of inheritance.
The diagnosis of OI largely depends on clinical presentation, including fragility fractures and family history, once secondary causes of osteoporosis have been excluded . Extraskeletal manifestations, such as blue sclerae, dentinogenesis imperfecta, and hearing loss, are also common in certain types of OI. A thorough examination, reviews of radiographs of long bones and skull, dentition evaluation, and detailed family history are the keys to making the diagnosis. Prenatal ultrasonography can sometimes detect severe OI as early as 16 weeks of gestation. However, clinicians often face challenges in making the diagnosis of OI in the absence of positive family history or apparent extraskeletal manifestations. In such situations, genetic analyses yield a rapid diagnosis in most cases.
Given the lack of definite treatment, symptomatic management by a multidisciplinary care team remains the mainstay of therapy for patients with OI. Rehabilitative care aims to optimize muscle function with ultimate goals to improve motor development, mobility, and functional independence . Orthopedic surgery for complex fractures and spinal fusion to stabilize severe scoliosis have been shown to improve ambulation and functional outcomes . Children and adolescents with mild forms of OI, however, are usually able to participate in high-level activities , although contact sports are not recommended.
Apart from bone morbidity, patients with OI, especially severe types, often suffer from cardiopulmonary complications such as recurrent pneumonia and cor pulmonale. Thoracic scoliosis greater than 60 degrees is strongly correlated with severely compromised pulmonary function . Valvular abnormalities and aortic diseases have also been reported to be associated with OI . Routine audiology evaluation is also recommended due to the high incidence of mixed conductive and sensorineural hearing loss after age 10 years . Neurologic complications, including basilar invagination, brain stem compression, hydrocephalus, and syringomyelia, have also been frequently reported in up to one-third of patients with OI. Therefore these patients require close monitoring, especially those with lower height Z -score . Lastly, bisphosphonate therapy, in conjunction with rehabilitative and surgical care, has been shown to improve the quality of life of children and adolescents with OI . Details of bisphosphonate therapy in OI will be discussed in the later section.
37.3.1.2
Osteoporosis pseudoglioma syndrome
OPPG is an autosomal-recessive disorder that shares similar bone phenotypes to moderate-to-severe OI, including low bone mass, short stature, and bone fragility. Unlike OI, patients with OPPG also have early-onset blindness due to persistent vitreous hyperplasia (pseudoglioma), corneal opacity, and secondary glaucoma . Cognitive impairment, ligamentous laxity, and hypotonia have also been observed . OPPG is caused by loss-of-function mutation of the low-density-lipoprotein receptor–related protein 5 ( LRP5 ) that results in impaired Wnt signaling that is critical for osteoblast differentiation and function . Heterozygous LRP5 mutations have been reported to be associated low BMD with preserved vision .
Severe osteoporosis is common in children and adolescents with OPPG. Short- and long-term bisphosphonate therapy has been shown to improve aBMD and alleviate bone pain . However, in some patients, fragility fractures continued to occur despite an increased BMD . pQCT measures in OPPG, both untreated or previously treated with bisphosphonate, have demonstrated reduced trabecular vBMD and cortical area, consistent with diminished bone formation, the key pathogenesis of OPPG . These findings highlight the limitation of DXA in predicting fracture risk. Teriparatide, a recombinant parathyroid hormone (PTH), has been used for treatment of severe osteoporosis in two young adults with OPPG with conflicting aBMD response . To date, bisphosphonate has remained the mainstay medical treatment for osteoporosis in children and adolescents OPPG, but there is a need for other therapeutic approaches to reduce fracture occurrence.
37.3.1.3
Idiopathic juvenile osteoporosis
IJO is a rare form of primary osteoporosis that usually presents in previously healthy children prior to puberty (mean age 7 years) with spontaneous recovery after adolescent years . Common presentations are insidious onset of pain in back and lower extremities, and abnormal gait preceding fragility fractures. Long bone fractures are frequently surrounded by radiolucent areas. There is generalized low aBMD and vBMD with more a pronounced reduction in the trabecular compartment .
The diagnosis of IJO is made after the exclusion of other forms of primary or secondary osteoporosis. Genetic analysis of genes associated with OI and LRP5 can be helpful to differentiate IJO from milder forms of OI or mutations in LRP5 gene given subtle differences in their initial manifestations. Bone histomorphometry from children and adolescents with IJO has demonstrated markedly reduced trabecular bone volume with low bone formation rate , in contrast to OI. Lastly, a history of spontaneous bone recovery after the pubertal onset suggests IJO rather than OI. Although a small number of patients with IJO continue to have persistent bone disease, there is the possibility that these patients may carry different disorders of which underlying mutation of genes regulating bone formation has not been yet identified.
Because spontaneous recovery is common, close monitoring and ensuring adequate vitamin D and calcium intake, and regular engagement in weight-bearing exercise are sufficient in most cases of IJO. Bisphosphonate use is currently limited to children and adolescents with IJO who suffer from significant bone pain or fragility fractures. Although bisphosphonate has been shown to improve aBMD and reduce fracture rates when compared with untreated patients , caution is needed as an atypical femur fracture (AFF) has been reported in a patient with IJO .
37.3.2
Secondary osteoporosis
37.3.2.1
Disuse osteoporosis
The concept of muscle strength in the developing skeleton or the “muscle-bone unit” is well described in children and adolescents . Increased muscle load and bone growth itself are the two important mechanical challenges that promote the development of bone mass and microarchitecture. Disuse osteoporosis is a term describing bone loss associated with mechanical unloading to that bone from chronic immobilization, often due to neuromuscular disease or spinal cord injury. Apart from a diminished muscle load on developing bone, leading to uncoupling bone remodeling, alterations in the effects of nervous system on bone cells, and bone marrow adipose tissue have been linked to the pathogenesis of disuse osteoporosis .
Cerebral palsy
Cerebral palsy (CP) is the most common nonprogressive motor disability in childhood with an estimated prevalence of 2 per 1000 live births . Children and adolescents with CP are prone to fragility fractures, especially in non-weight bearing patients , and the odds of fractures are higher among patients with height for age <−3 SD or on antiepileptic therapy . A systematic review showed an annual fracture incidence of 4% in children with and adolescents with nonambulatory CP , with over 75% of fracture occurrence in the lower extremities . Most fractures also arise without apparent preceding trauma .
In conjunction with bone fragility, low BMD is common in patients with CP . The pathogenesis of low BMD in CP is largely due to chronic immobilization and diminished mechanical loading upon developing bone . The degree of preserved weight-bearing has been shown to be positively correlated with BMD . LS BMD has failed to predict the risk of fracture in a study of children and adolescents with quadriplegic CP . In contrast, LDF BMD has been found to be strongly associated with fracture risk in moderate-to-severe CP and has become a recommended site of DXA measure in children and adolescents with CP . Similar findings on axial and peripheral BMD have been noted in a study of ambulatory children with CP using QCT measurements . While ambulatory status appears to play a key role in predicting risk for osteoporosis in CP, there are conflicting results on the impact of weight-bearing activity intervention on BMD . In studies identified improvement of BMD, there was subtle increase (2%–6%) in BMD at various sites of measurement , although a longitudinal study of 69 children with spastic CP has demonstrated spontaneous increased BMD at 2%–5% annually, without intervention . Furthermore, there are no studies evaluating the influence of such physical therapy intervention on fracture outcome in children and adolescents with CP.
Nutritional status, as determined by BMI, skinfold thickness, and height for age, have also been found to be important determinants of bone health in CP . Although the serum 25-hydroxyvitamin D level was not correlated with BMD , optimizing vitamin D and calcium intake remains essential as vitamin D deficiency is prevalent in this patient population , and supplemental vitamin D and calcium may have a positive impact on bone mineralization in children and adolescents with CP receiving antiepileptic drugs (AED) .
A recent systematic review of eight studies of intravenous (IV) bisphosphonate therapy in children and adolescents with CP (six pamidronate, one risedronate, and one zoledronic acid) has shown significant gain in aBMD at LS, total body and region 1, but not three of the distal femur following 6–18 months of treatment . In studies reported fracture outcome, it appears that bisphosphonate may reduce the fracture rate .
In summary, bone health in children and adolescents with CP has remained an important area of future research. Current literature suggests multidisciplinary preventive and therapeutic approaches to lessen bone comorbidity and improve the quality of life in these patients.
Duchenne muscular dystrophy
DMD is an X-linked recessive disorder of progressive muscle weakness due to mutations in the dystrophin gene, with an estimated prevalence of 1 in 5000–7000 live male births . Progressive myopathy historically results in loss of ambulation by the early adolescent years and early death in late teens or early 20s from cardiopulmonary complications. However, early diagnosis and aggressive medical interventions have led to improvement in survival, with a mean age at death from respiratory complication increased to 27.9 years in DMD patients with mechanical ventilation .
Osteoporosis is a common secondary complication of DMD due to progressive muscle weakness, chronic inflammatory response to dystrophin-deficient muscle, chronic glucocorticoid therapy, and delayed puberty . Glucocorticoid therapy is the mainstay pharmacologic treatment modality for DMD as it has been shown to improve motor function, pulmonary function, delay loss of ambulation and increase overall outcome . Deflazacort has been increasingly used due to its less unfavorable side effects on weight gain when compared with prednisolone . Findings from studies in children with juvenile arthritis and those with postrenal transplant also favor deflazacort use because if its bone-sparing effects . However, relatively larger dose of deflazacort treatment in DMD may contribute to high incidence of osteoporosis in patients with DMD treated with deflazacort . Vertebral and long bone (mainly femur or tibia) fractures have been reported up to 30%–75% in patients with DMD . Concurrent glucocorticoid therapy was associated with fracture risk independent of ambulation status , and higher cumulative dose of glucocorticoid was negatively correlated with aBMD . Aligned with these findings, a longitudinal study of 41 children with DMD has shown declined LDF aBMD even when ambulation was minimally affected, though further reduction of bone mass was observed with loss of ambulation .
The limitations of DXA as a screening tool for osteoporosis is apparent in DMD as many vertebral and long bone fractures occur with aBMD Z -score above −2.0 . Skeletal sites also appear to affect the interpretation of DXA. A large observational study of 292 glucocorticoid-treated youth with DMD has shown an LS aBMD to correlate poorly with bone health outcome . Furthermore, vertebral fracture in children treated with glucocorticoids can be asymptomatic. Routine spine radiograph has been shown to early detect asymptomatic vertebral fractures in DMD , which is relevant in pediatric population because the presence of asymptomatic or mild vertebral fracture is predictive of future fracture . The DMD Care Considerations Working Group suggests lateral spine radiographs for vertebral fracture detection in patients with back pain, and at baseline with routine periodic follow-up X-ray (every 1–2 years for glucocorticoid treated, every 2–3 years for untreated) in all asymptomatic patients . Baseline DXA scans are also recommended as it remains a useful tool to monitor bone health trajectory and response to bone health intervention.
Vitamin D deficiency is common in children and adolescents with DMD, despite chronic supplementation . Optimizing vitamin D and calcium intake has been shown to impact positively on bone health as evident by normalized bone turnover and improved BMD . Bisphosphonate therapy is generally preserved for patients with evidence of fragility fractures. Moderate-to-severe asymptomatic vertebral fractures may warrant treatment because spontaneous vertebral reshaping has not been observed in DMD . IV bisphosphonates (pamidronate or zoledronic acid) have been shown to help with bone pain, stabilize or improve vertebral height, and improve BMD in children with DMD with symptomatic vertebral fracture . Data on oral bisphosphonate (alendronate) are also available in this population but with conflicting results . The efficacy of bisphosphonate in preventing future fracture in DMD has not been examined by an adequately powered study. Use of other agents for the treatment of osteoporosis in DMD has been reported in two patients—teriparatide in a 20-year-old male and denosumab in a 13-year-old boy . Improvement of aBMD was observed in both reports.
To date, IV bisphosphonates have remained the first-line pharmacologic therapy of osteoporosis in children and adolescents with DMD . Future studies are needed to investigate the efficacy of bisphosphonate and newer anabolic agents in preventing further fracture in this population.
Spinal muscular atrophy
Spinal muscular atrophy (SMA) is a progressive neuromuscular disorder characterized by spinal motor neuron degeneration due to homozygous mutations in SMN1 gene. The estimated prevalence of SMA is 1 in 6000–10,000 live births . SMA is categorized by severity with type 1 being the most severe type with infantile onset and type 4 being the mildest type with adult onset of mild muscle weakness . Progressive muscle weakness commonly leads to chronic immobilization and potentially disuse osteoporosis. The prevalence of fracture in patients with SMA has been reported from 9.3% to 46% and fracture occurs mostly in the lower extremities . A recent study of 85 children with SMA has revealed that fracture is common regardless of the severity of SMA . Although 85% of these patients had an aBMD Z -score less than −2.0 at any scan site, only 13% fulfill the criteria for pediatric osteoporosis. Bisphosphonate has been used for treatment of osteoporosis in children and adolescents with SMA, mainly included in cohorts of disuse osteoporosis . However, to date, there has not been a study of long-term bone health outcome following bisphosphonate therapy in this population.
37.3.2.2
Chronic diseases associated with osteoporosis
Cystic fibrosis
Cystic fibrosis (CF) is an autosomal-recessive disorder caused by mutations in CFTR gene. A defect in chloride channel leads to early respiratory failure and pancreatic insufficiency. Advances in multidisciplinary care for CF have led to an increased life expectancy, and CF-related bone disease has become recognized as a complication in CF. A cross-sectional study of adolescents and young adults with CF has shown that they are at ninefold increased risk of fractures when compared with healthy controls, and 60% are likely to sustain any fracture by age 25 years .
Chronic inflammation, undernutrition, vitamin D deficiency, physical inactivity, delayed growth and puberty, and glucocorticoid therapy are contributing factors for poor bone health in children and adolescents with CF . A retrospective correlation analysis of 60 young patients with CF and normal linear growth has demonstrated the poorest bone mass accrual, as measured by change in LS aBMD, in those with low BMI and poor pulmonary function . Low aBMD at various skeletal sites in children with CF has been demonstrated in numbers of studies . Children with CF also have lower total BMD, cortical thickness and lower strength index of the distal radius, and lower LS and TBLH BMD when compared with controls . Trabecular bone deficits are also demonstrated in tibia . Alteration of bone architecture by pQCT measurements suggest that low aBMD in this population is likely true as pQCT is not affected by small bone size. Bone health parameters obtained by DXA and pQCT have high negative predictive value . To date, there is only one study using HRpQCT to assess bone microarchitecture in children and adolescents with CF. Bone microarchitecture is minimally affected in children with CF who have relatively reserved pulmonary function and nutritional status .
Current recommendations suggest obtaining baseline DXA scans as part of bone health screening in children with CF, as early as age 8 years of age, with ideal body weight <90%, FEV1 <50% predicted, chronic glucocorticoid exposure, and a history of fracture . However, caution is needed when interpreting DXA in children with CF as small bone size could overly estimate the deficit in aBMD. Apart from nutritional evaluation, children and adolescents with CF should undergo routine pubertal and growth assessment as part of bone health evaluation and appropriate interventions, such as ensuring adequate vitamin D and calcium intake should be employed. Sex hormone replacement in adolescents with delayed puberty can be considered on an individualized basis. Minimizing glucocorticoid use is also of importance to prevent further bone loss. A systematic review of seven trials of bisphosphonates (oral and IV) use in adults with CF has shown an improved aBMD, but these studies are not powered enough to demonstrate the effect on fracture incidence . There is only one study of bisphosphonate use in children and adolescents with CF . There was a significantly greater increase in aBMD in alendronate-treated group when compared with placebo group. To date, there are no studies evaluating bisphosphonate and fracture outcome in pediatric population with CF. Current guideline suggests that bisphosphonate therapy could be considered in children with significant fragility fractures . In the absence of fractures, children undergoing organ transplantation or on chronic glucocorticoids may be justified for bisphosphonate treatment.
Celiac disease
Celiac disease (CD) is an immune-mediated intestinal disorder characterized by gluten-sensitive enteropathy. The estimated prevalence of CD in North America and Europe is up to 1% . Abdominal pain, weight loss, and anemia are common symptoms of CD, but they can be subtle and delayed growth and puberty and reduced bone mass can be the only findings. A reduced aBMD is common in children and adolescents with CD at the time of diagnosis . In a large population-based study, CD in children and adolescents was also associated with hip fracture and fracture at any other site with hazard ratio of 2.6 and 1.1, respectively . The metabolic bone consequences of CD are results of malabsorption and fecal loss of calcium and vitamin D due to enteropathy, and impaired bone formation and increased bone resorption from chronic inflammation . Common autoimmune disorders associated with CD such as type 1 diabetes mellitus (DM) and hypothyroidism can also contribute to further reduction in bone mass.
Gluten-free diet and addressing nutritional needs are pivotal in management of CD. A cross-sectional study of 81 children with CD has shown a higher mean LS aBMD in those who were treated with gluten-free diet for 2 years when compared with an untreated group . Rapid normalization of aBMD and BMC by 6–12 months with strict gluten-free diet has been observed in many longitudinal studies . A study in adults with CD has also demonstrated that fracture risk also is significantly reduced with strict gluten-free diet . Adequate calcium and vitamin D intake should also be ensued as avoidance of dairy products is commonly reported in children and adolescents with CD . Expert panel recommends routine screening for vitamin D deficiency at the time of CD diagnosis and considering DXA scans in selected children and adolescents who are not adherent with gluten-free diet . Routine densitometry is not recommended unless there is clinical concern for bone pain or osteomalacia. At present, data on bisphosphonate therapy for children and adolescents with CD are lacking.
Inflammatory bowel diseases
About 20% of patients with Crohn disease and 12% of ulcerative colitis present prior to age 20 years . Both diseases are characterized by chronic inflammation and malnutrition, which contribute to delayed growth and puberty, altered body composition, and low aBMD . Skeletal deficits are evident from diagnosis as bone histomorphometry in children with newly diagnosed inflammatory bowel disease (IBD) showed evidence of low bone formation and bone resorption . Furthermore, the age of onset also influenced the extent of compromised bone health. A retrospective study of postmenopausal women with IBD has demonstrated that women who presented at age <16 years had lower LS and hip aBMD and BMC than those who were diagnosed at older age . Low aBMD appears to persist even after adjusted with height Z -score . Although there is clear evidence of increased fracture risk in adults with IBD , population-based studies in children and adolescents are lacking. Data from a study of 132 children with IBD showed no significant difference in long bone fracture risk when compared with their healthy siblings . Fracture risk in children with IBD was also similar to healthy controls in a large cross-sectional study . Vertebral fractures with low aBMD have also been reported in pediatric IBD and with and without glucocorticoid therapy .
Bone threats in children and adolescents with IBD include chronic inflammation, delayed growth and skeletal maturation, inadequate physical activity, vitamin D deficiency, and glucocorticoid therapy . Low lean body mass is also common in this population and has also been linked to low aBMD . Current recommendations suggest annual monitoring of serum 25-hydroxyvitamin D level and ensuring adequate vitamin D and calcium intake . BMD screening and monitoring is also recommended in high-risk patients, including those with poor linear growth, low BMI, delayed puberty, severe inflammatory disease, and prolong glucocorticoid therapy >6 months . Successful treatment of IBD can lead to improved BMD and vertebral reshaping . There are limited data on bisphosphonate use in children and adolescents with IBD. In two cohorts of osteoporosis associated with glucocorticoid therapy or chronic inflammation that included children and adolescents with IBD, there was modest improvement of LS aBMD and vBMD following bisphosphonate therapy . Further studies are needed to determine the indication, and safety and efficacy of bisphosphonate therapy in pediatric IBD.
Rheumatologic disorders
Pediatric rheumatologic diseases, such as systemic lupus erythematosus (SLE), juvenile idiopathic arthritis (JIA), scleroderma, and juvenile dermatomyositis (JDM), share common characteristics of chronic inflammation that leads to compromised bone health . Weight-bearing activity is often limited due to pain and weakness. In addition, glucocorticoids, which are known to have deleterious effects on bone health, are often used for acute and chronic treatment of these disorders. As a result of these factors, reduced BMD and fractures are common complications of rheumatologic disorders in children and adolescents.
Low aBMD and vBMD and poor bone geometry at various skeletal sites, independent of glucocorticoid treatment, have been noted in children and adolescents with JIA when compared with healthy controls in multiple cross-sectional studies . Similarly, reduced bone mass is well documented in juvenile SLE and JDM . The negative impacts of rheumatologic diseases on bone health are not only observed in children and adolescents but also in adults, as few studies have found that JIA and juvenile SLE could be associated with reduced BMD in adulthood. The altered bone mass and geometry plausibly account for increased incidence of long bone and vertebral fractures in children and adolescents with rheumatologic diseases. A population-based study of patients with juvenile-onset arthritis has shown increased risk of fractures throughout childhood into adulthood . Vertebral fractures are reported in 7%–22% of children with rheumatologic diseases . However, the true incidence of vertebral fractures in this population is likely higher as asymptomatic vertebral fractures were not often actively screened. It should be noted that vertebral fractures occur even prior to glucocorticoid exposure.
Minimizing glucocorticoid use is an optimal measure to prevent and alleviate bone loss, although commonly unfeasible prior to the era of biologic therapies. Inadequate intake from poor appetite is common children with rheumatologic diseases due to chronic inflammation. Therefore calcium and vitamin D intake should be evaluated and optimized. The guideline for prevention and treatment of glucocorticoid-induced osteoporosis (GIO) published by the American College of Rheumatology in 2017 suggests to consider bisphosphonate therapy, plus calcium and vitamin D, for treatment of fragility fractures in children age 4–17 years who are on chronic glucocorticoids ≥0.1 mg/kg/day for ≥3 months . These recommendations are conditional since there are limited data on safety and efficacy of bisphosphonate therapy rheumatologic disorders. A systematic review of oral and IV bisphosphonate use in children and adolescents JIA has demonstrated a modest increase in LS aBMD ranging from 4.5% to 19.1% . One randomized control trial that included 13 young patients with rheumatologic diseases has found a significant gain in LS aBMD in alendronate treated, but not placebo group, and no new fracture was observed . Improvement of vBMD by QCT was also observed in one study of patients with JIA treated with oral clodronate . Variation in bisphosphonate type, route, dosage and duration, and small numbers of participants limits the power of these studies to study fracture outcome. At present, there is insufficient evidence to support routine use of bisphosphonates for children and adolescents with rheumatologic diseases, and its use for treatment of fragility fracture is largely on a compassionate care basis .
Renal disease
Extrarenal complications of chronic kidney disease (CKD) in children and adolescents often include growth failure, delayed puberty, reduced muscle mass, and change in bone and mineral metabolism. CKD–mineral and bone disorder (CKD–MBD) is the broad term describing systemic change in bone metabolism associated with CKD. The pathogenesis of CKD–MBD is complex. As kidney function declines, the ability of nephrons to excrete phosphate diminishes. Hyperphosphatemia results in elevated PTH, decreased 1,25-dihydroxyvitamin D, and increased FGF-23 . In addition, impaired conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D further decreases intestinal calcium absorption and increases PTH level. The alterations of bone histomorphometry due to the aforementioned abnormal mineral metabolism in CKD–MBD can be categorized into (1) osteitis fibrosa cystica, (2) adynamic bone disease, (3) osteomalacia, and (4) mixed osteodystrophy, and the severity of these alterations rises with worsening glomerular function . Greater detail of CKD–MBD can be found in Chapter 54, Osteoporosis associated with chronic kidney disease. A large perspective cohort study has found that children with CKD have two to three times greater risk of fracture at any site than general population . In multivariable correlation analysis, advanced pubertal stage, difficulty walking, and higher PTH level were associated with greater fracture risk.
Although many studies have used DXA for assessment of bone health in pediatric CKD, delayed growth and puberty can influence aBMD measurement by DXA. Furthermore, the key skeletal changes in CKD–MBD may not be captured by DXA. The differential effects of hyperparathyroidism on bone compartments have been well demonstrated by pQCT . Hyperparathyroidism leads to cortical bone loss while stimulating trabecular bone formation in children and adolescents with CKD when compared with healthy controls. A study evaluating DXA measures in children with CKD found only modest correlation between DXA and pQCT measures in this patient population .
Treatment of CKD–MBD aims to prevent hyperphosphatemia, hypocalcemia, and hypovitaminosis D—all of which contribute to secondary hyperparathyroidism, a universal finding of progressive CKD. Hyperparathyroidism can occur when glomerular filtration rate (GFR) is <90 mL/min per 1.73 m 2 , but obvious bone changes are not typically observed until GFR <50 mL/min per 1.73 m 2 . Given these observations, current recommendation suggests monitoring of bone and mineral metabolism markers (serum calcium, phosphorus, PTH, and alkaline phosphatase) in children, beginning in CKD stage 2 (GFR between 60 and 89 mL/min per 1.73 m 2 ) . Early detection and intervention of these metabolic abnormalities are essential to prevent or minimize skeletal complications of CKD–MBD. Although, rare in pediatrics, parathyroidectomy may be required in severe secondary hyperparathyroidism or tertiary hyperparathyroidism. The limited data on bisphosphonate therapy for children and adolescents receiving glucocorticoids or posttransplantation are discussed elsewhere in this chapter.
37.3.2.3
Hematologic and oncologic diseases
Hemoglobinopathies
Hemoglobinopathies is a group of red blood cell disorders that is characterized by reduced red blood cell life span and anemia. Depending on disease severity, patients with thalassemia and sickle cell anemia (SCA) may require regular blood transfusion as well as chelation therapy for treatment of iron overload. Common non-hematologic comorbidities of thalassemia major include delayed growth and puberty, hypothyroidism, bone pain, and osteoporosis . SCA is also associated with growth and puberty delay and low bone mass, but not other endocrinopathies seen in thalassemia . Nutritional deficiency, growth delay, hypogonadism, and reduced lean body mass are all detrimental to bone health. In addition, increased bone marrow turnover and chronic anemia and chronic inflammation further implicate skeletal deficits in hemoglobinopathies .
Although limited data have not found an increased incidence of fractures in children and young adults with thalassemia or SCA , reduced aBMD and BMC at various skeletal sites have been well demonstrated in these patients regardless of their transfusion status . A large cross-sectional study of children and adolescents with thalassemia has revealed worsened LS and femoral neck aBMD Z -score during adolescence years, suggesting that bone accrual is lagging in these patients when compared with age-matched peers . Lower body weight and hypogonadism were negatively correlated with BMD. Fractures history was reported in 36% of patients and was associated with low BMD. However, caution is needed when interpreting DXA results as growth and puberty delay are common in this patient population, similar to other chronic diseases. Furthermore, recent studies have identified that hepatic iron overload in patients with thalassemia and SCA could falsely elevated aBMD value, especially in L1 region . The skeletal deficits have also been validated using pQCT in young patients with thalassemia as tibial cortical area, BMC, and thickness were significantly lower when compared with healthy controls .
Optimizing nutrition and physical activity are the mainstay of treatment for poor bone health associated with hemoglobinopathies. Hormone therapy such as thyroid and sex hormone replacement should be provided to patients with thalassemia who are deficient. To date, there is no sufficient date to evaluate the safety and efficacy of bisphosphonate therapy for treatment of fragility fractures in children and adolescents with hemoglobinopathies.
Childhood malignancy
Given an increase in overall survival rate, long-term sequelae of childhood cancer have become a focus of attention in care of childhood cancer survivors. Children and adolescents with cancer are at risk of reduced BMD and fractures, as a result of disease processes and its treatment that often include glucocorticoids . In order to prevent and treat skeletal deficits associated with childhood malignancy, all patients should be encouraged to optimize calcium and vitamin D intake and also be monitored for endocrine deficiencies. Current recommendations suggest baseline BMD measurement at the time of entry into long-term follow-up (usually occurs 2 years after completion of chemotherapy) in children with predisposing factors to BMD reduction such as prior glucocorticoid exposure, cranial radiation, methotrexate use, or hematopoietic cell transplantation (HCT) . Follow-up DXA scans may be warranted in children and adolescents with significantly reduced BMD, fragility fractures, or endocrine deficiencies.
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy. Skeletal abnormalities, including pain, abnormal gait, and radiographic changes such as osteopenic appearance, have been well documented in children with ALL at diagnosis . Moreover, alterations of bone metabolism markers and osteomalacia findings from bone histomorphometry have also been observed at diagnosis . Bone insults from leukemic cell infiltration, inflammation, and paraneoplastic factors prior to treatment of ALL likely contribute to prevalent long bone and vertebral fractures at diagnosis . A large prospective cohort of children with ALL has reported vertebral fractures in 16% of patients at diagnosis . The decline in aBMD at trabecular and cortical bone sites was observed as soon as 6 months from the initiation of ALL therapy and is associated with older age of onset ≥10 years and single-nucleotide polymorphisms in COL11A1 and NELL1 genes on the genome-wide analysis study . A global reduction in both bone compartments was confirmed by studies using pQCT . The peak bone morbidity occurs at 12 months of treatment and manifests by 24 months in most cases . The cumulative incidence of vertebral fractures increased to 26% at 4 years , and 40% of these fractures were asymptomatic . Baseline vertebral fracture, regardless of severity, baseline LS BMD Z -score, and glucocorticoids exposure >10 mg/m 2 /day of prednisone were the strongest predictors long bone and vertebral fractures risk in these children. Although spontaneous skeletal recovery and improvement of BMD have been observed , persistent vertebral deformity following fractures was noted in nearly 25% of patients . While glucocorticoid therapy has remained a culprit of skeletal deficits in childhood ALL, other chemotherapeutic agents such as asparaginase and methotrexate are also known to have deleterious effects on growing skeleton . Furthermore, ALL survivors continue to face other bone threats such as hypothyroidism, hypogonadism, and growth hormone (GH) deficiency . Promoting adequate calcium and vitamin D intake in survivors of childhood ALL is an essential measure to promote bone health as their intake is often inadequate . Even though oral and IV bisphosphonate appeared to improve BMD in young patients with ALL in small studies , medical therapy with bisphosphonate for treatment of fragility fractures and osteoporosis in children and adolescents with ALL has remained considered compassionate use, similar to other pediatric osteoporotic conditions, as there are no available control trials to evaluate its efficacy in reduction of bone loss or fractures risk in this patient population.
Reduced BMD is also observed in children and adolescents with brain tumors, especially in those who had received cranial irradiation . Direct effects of the irradiation, GH and gonadotropins deficiency, and reduced physical activity are thought to be contributing factors of skeletal deficits in these patients. Similarly, children and adolescents undergoing treatment for solid tumors were also found to have BMD reduction .
Transplantation
Increased long-term survival after HCT and solid organ transplantations from advances in medical care have led to an emerging long-term complication, including bone fragility . Bone fragility is evident in pediatric transplant survivors. A prospective population-based study of children and adolescents undergoing solid organ transplantation has observed fragility fracture in 38% of patients, and the risk of vertebral fracture was 160-fold higher than the control population . Skeletal deficits following transplantation are attributed to direct effects of chronic disease or malignancy, graft-versus-host disease, poor nutrition, reduced physical activity, and exposure to cytotoxic agents, glucocorticoids, and irradiation. The diagnosis and management of osteoporosis in adults with organ transplantation are reviewed in Chapter 52, Osteoporosis in organ transplant patients.
HCT refers to the administration of autologous or allogeneic hematopoietic progenitor cells derived from peripheral blood or bone marrow to reconstitute the bone marrow in patients with malignancy, bone marrow failure, immunodeficiency, autoimmune diseases, and inborn errors of metabolism. Several studies have found reduced BMD in pediatric patients undergoing HCT by DXA and QCT with greater skeletal deficits when compared with patients undergoing chemotherapy alone . Bone loss was observed as early as 30 days following HCT . Female sex, prepubertal age at HCT, GH deficiency, and total body irradiation have been identified as risk factors of skeletal deficits in these studies. Most recommendations suggest baseline DXA scans at 1 year following HCT or sooner with other risks factors . One prospective study has demonstrated persistent aBMD reduction up to 9 years following pediatric HCT .
Similar findings of skeletal deficits have been demonstrated in pediatric patients undergoing solid organ transplantation . Studies evaluating bone histomorphometric changes following solid organ transplantation have shown heterogeneity of histological findings, including low trabecular volume, abnormal turnover, and altered bone mineralization . These studies are limited by relatively smaller size of participants and variability in types of transplantation.
Prevention and treatment of skeletal deficits following transplantation include optimizing nutrition, calcium, and vitamin intake; increasing physical activities; and minimizing use of medications causing bone threat such as glucocorticoids. Periodic clinical evaluation of hormone deficiencies, including thyroid, sex steroids, and GH should be performed.
A meta-analysis of nine studies has shown that bisphosphonate or vitamin D was effective in reduction of fracture risk in adults undergoing solid organ transplantation . Routine use of bisphosphonate and/or vitamin D following transplantation to prevent osteoporosis has been recommended in adults with anticipated prolonged course of glucocorticoids, although there is no standard protocol regarding the treatment regimen . Less is known about the risks and benefits of bisphosphonate therapy in children and adolescents post-transplantation. A randomized controlled trial in 60 children post-renal transplantation has shown improvement in aBMD Z -score in alendronate-, alfacalcidol-, or nasal calcitonin–treated group while untreated controls had further declined Z -score . Another study included 17 children and adolescents undergoing solid organ transplantation and HCT in a group of 29 patients with GIO . There was a trend of improved aBMD at 1 year of IV bisphosphonate therapy. These studies were not adequately powered to determine fracture outcomes. Currently, there is insufficient evidence to support routine use of bisphosphonate following transplantation in children and adolescents.
37.3.2.4
Endocrine and reproductive disorders
Diabetes mellitus
DM has been shown to have a negative impact on bone health in adults, evident by reduced bone mass in type 1 DM and increased risk of fractures in type 1 and type 2 DM . However, the impact of DM on growing skeleton in children and adolescents with DM is less certain. A slight increase in fracture risk in children and adolescents with type 1 DM when compared with healthy controls has been demonstrated in a population-based study—hazard ratio 1.14 and 1.35 in males and females, respectively . While many studies have demonstrated a reduction of axial and appendicular bone mass , no significant aBMD loss has been observed in several studies . Studies using QCT or pQCT have noted diminished vBMD, cortical BMC, and bone area . These conflicting results probably reflect variability of sample sizes, DM duration, and glycemic control. Poor glycemic control has been linked to skeletal deficits in some studies , but not by others . The proposed mechanisms by which type 1 DM negatively affects bone health include impaired bone formation from chronic hyperglycemia and insulin deficiency, alteration of bone strength from advanced glycation, and urinary loss of calcium and phosphate from glucosuria . Theoretically, late complications of DM could negatively influence bone health, for example, CKD from nephropathy, and decreased physical activity from neuropathy or retinopathy.
While BMD is preserved in adults with type 2 DM , several studies have shown increased cortical porosity that may contribute to increased fracture risk in these patients . Less data are available in youth with type 2 DM, but many studies have demonstrated the negative impact of insulin resistance on bone health .
In summary, DM may be associated with reduced bone mass in children and adolescents but its clinical importance remains controversial. Currently, routine bone health screening is not part of standard of care in youth with diabetes . However, optimizing glycemic control to prevent advanced diabetic complications may have a long-term positive impact on bone health.
Growth hormone deficiency
GH and insulin-like growth factor 1 (IGF-1) are essential for bone mass acquisition in children and adolescents . IGF-1 directly regulates longitudinal bone growth by stimulating osteoblast proliferation and bone matrix production . Furthermore, the effect of GH and IGF-I on lean body mass attributes to bone strength .
Low BMC and aBMD, corrected for delayed bone age and small bone volume, at various skeletal sites have been observed in children and adolescents with GH deficiency in several , but not all studies . However, the impact of low BMD on fracture risk in these young patients has not been well established. A study of 46 children with GH deficiency has demonstrated the association between fracture and low LS aBMD corrected for bone area . One year of GH therapy in prepubertal children has been shown to be associated with gain in bone area , and longer treatment has been showed to improve aBMD and BMC to normal range . Adults with childhood-onset GH deficiency are at higher risk of fracture , and GH replacement have been shown to improve aBMD and decrease fracture risk . Therefore continuing GH replacement through late adolescence and adulthood may be of benefit in these patients.
Thyroid disorders
Excess thyroid hormone results in simulation of osteoclastic bone resorption . Resultant increases in serum calcium and phosphate levels lead to decreases in seum PTH and 1,25-dihydroxyvitamin D levels. As a result, intestinal calcium absorption decreases and urinary calcium excretion increases. The net effect is skeletal deficits and bone fragility. Greater details of the effects of thyroid hormone on bone health are discussed in Chapter 46, Thyroid hormone, thyroid medication, and the skeleton.
There are limited data of the effects of hyperthyroidism on growing skeleton. A prospective study of 26 children and adolescents with Graves’ disease has demonstrated significantly reduced aBMD with greater deficit at cortical bone site . A study using QCT has confirmed cortical bone as preferential site of bone loss in hyperthyroidism . Fragility fractures have also been reports in pediatric Graves’ disease . Successful treatment of hyperthyroidism with antithyroid drugs appeared to improve aBMD . Reduced bone mass has also been observed with high-dose levothyroxine treatment in children with goiter or thyroid cancer and severe congenital hypothyroidism . Therefore the benefits of over suppression of thyroid-stimulating hormone and bone health risks should be carefully considered in these patients.
Hypogonadism
Sex steroid plays a critical role in bone metabolism from young age through adulthood . Estrogen is essential for bone maturation and bone mass accrual in both males and females . Impaired estrogen synthesis in men with aromatase deficiency is associated with reduced BMD and delayed bone maturation, and estrogen replacement therapy (ERT) has been shown to improve skeletal deficit and advance epiphyseal closure . Androgen also has a positive impact on bone through the development of muscle mass that stimulates periosteal apposition leading to increased cortical thickness and bone diameter . The onset of pubertal maturation also plays an essential role in bone mass accrual . Delayed puberty has been linked to low aBMD and BMC at various skeletal sites . Skeletal deficits have been confirmed by studies using pQCT that showed low trabecular bone mass and increased risk of fractures in patients with a history of late-onset puberty . Interestingly, short-term testosterone therapy, which is often used in boys with delayed puberty, has not been showed to affect peak bone mass .
Delayed pubertal onset should prompt a thorough evaluation to distinguish idiopathic from pathologic delay. Sex steroid deficiency can be manifestation of Turner syndrome (TS), malnutrition, eating disorders, and exercise-induced amenorrhea or complications of childhood malignancy, transplantation, and other chronic diseases. Permanent hypogonadism warrants long-term sex steroid replacement. However, there is a lack in consensus regarding optimal hormone formulation, route, and dose in many forms hypogonadism. Discussion on ERT in TS can be found in the following section. Chapter 44 discusses eating disorder in greater detail.
Turner syndrome
Short stature and delayed puberty are common manifestations in girls with TS. Bone fragility has been recognized as a significant comorbidity of TS based upon previous studies that showed increased risk of fractures across all ages despite normal BMD . Nevertheless, data from a recent US national survey have demonstrated that the prevalence of fracture in young patients with TS (<25 years) did not differ from control group, but the forearm fractures were more frequently reported . Risk factors associated with fractures in TS include older age, history of parental fracture, hearing impairment, balance problem, and low BMD . Although reduced aBMD by DXA is commonly reported in children and adolescents with TS, it is likely confounded by small bone size and delayed puberty. To address these challenges, several studies have used QCT or pQCT, although with variable results—reduced vertebral trabecular BMD only , reduced cortical bone area or vBMD with preserved trabecular BMD of radius . Recent findings from a study of 32 adolescents with TS have suggested that low cortical vBMD from previous studies were probably falsely reduced due to partial volume effect . Lastly, several studies have demonstrated compromised bone microarchitecture in young patients with TS using HRpQCT .
GH to promote linear growth and ERT for treatment of ovarian failure have become standard of care for children and adolescents with TS . Not only ERT helps to initiate puberty and maintain secondary sex characteristics, but it has also been shown to preserve BMD in TS . ERT should begin at age 11–12 years with slow titration to full adult replacement dose in 2–3 years . Transdermal ethinyl estradiol is a preferred ERT as it has been shown to have superior effect on BMD in patients with TS , as well as other conditions with hypogonadism such as functional hypothalamic amenorrhea . Current practice guideline recommends bone density monitoring by DXA scans after ERT has been instituted, and screening for vitamin D deficiency every 2–3 years starting from age 9–11 years as 25-hydroxyvitamin D is often low in patients with TS . Future longitudinal studies of bone health trajectory are needed to determine best practice to optimize bone health outcomes in children and adolescents with TS.
37.3.2.5
Iatrogenic causes
Antiepileptic drugs
AED is the mainstay treatment of childhood epilepsy. The influence of AED on growing skeleton has remained debated. While data from adults have shown the association between AED use and increased fracture risk , there are insufficient fracture data in ambulant children treated with AED. In one study, ambulant adolescents treated with AED reported more fracture than their age-matched controls . Low aBMD in children treated with AED has been observed in some , but not all studies . It should be noted that most of these studies did not adjust aBMD for height. In several studies using pQCT, children treated with AED were found to have reduced trabecular vBMD at appendicular sites .
Older generation AED, including phenobarbital, phenytoin, and carbamazepine, induces many cytochrome P450 enzymes, thereby reducing vitamin D bioavailability. However, studies of noninstitutionalized ambulant children treated with enzyme-inducing AED did not report fracture or clinical rickets . In summary, routine BMD screening in ambulant children treated with AED without other osteoporotic risk factors is not warranted. However, periodic assessment of vitamin D status should be considered in children with older generation AED.
Aromatase inhibitors
Aromatase inhibitors have been studied in attempts to improve final adult height in children and adolescents with short stature by inhibiting conversion of testosterone to estrogen, thereby delaying epiphyseal closure. Off-label uses of aromatase inhibitors have also been reported in children and adolescents with other growth and puberty disorders, such as aromatase excess syndrome, Peutz–Jeghers syndrome, McCune–Albright syndrome, and testotoxicosis . A meta-analysis of four randomized controlled trials in boys with short stature has shown improvement in short-term growth outcome but not final adult height . Due to bone health concern as men with aromatase deficiency have profound osteoporosis, these studies also evaluate bone health as secondary outcome. There was a trend of increased aBMD following 1–2 years of treatment similar to placebo group. However, vertebral abnormalities following treatment were noted in 45% of patients in one , but not other cohorts . The adverse skeletal effects and probable benefits of aromatase inhibitors should be thoroughly considered. Recommendation for bone health monitoring includes annual screening for vitamin D deficiency, bone age X-ray, bone density, and lateral spine radiographs .
Hormonal contraceptives
Hormonal contraceptives have variable effects on growing skeleton, depending on formulation and route of administration . The progestin-only contraceptives cause suppression of estrogen production and thereby could have a negative impact on bone mass accrual in adolescents females. Most studies have found depot medroxyprogesterone acetate (Depo-Provera, DMPA) use to be associated with an increased risk of bone loss . Although return to baseline aBMD following discontinuation of DMPA has been observed , this reversal may be insufficient as adolescents are expected to gain bone mass over those critical years . A large case–control study from the United Kingdom has demonstrated an increased fracture risk in women age 20–40 years with DMPA use . Younger age (<30 years) and longer duration of DMPA exposure (≥10 prescriptions) were associated with the highest risk of fracture. Fracture data in adolescents are limited. A cross-sectional study of 83 adolescents female with DMPA use did not find an association between fracture and DMPA use or baseline or subsequent aBMD . The authors concluded that fracture history is not an absolute contraindication for DMPA use.
While studies of skeletal health among adults have found neutral or positive effects of the oral forms of combined hormonal contraceptives (CHC, containing estrogen and progestin) on bone mass , this may not be true in adolescents whom often prescribed oral CHC with lower amounts of estrogen. Several longitudinal studies have demonstrated that adolescent girls using low-dose ethinyl estradiol (≤30 µg daily) had a significantly less aBMD gain when compared with non-CHC users . Another longitudinal study found no significant negative effects of oral CHC on BMD unless there were exposures for more than 6 years after the onset of peak height velocity . Data on the effects of other routes of CHC (vagina ring or patch) on bone health are lacking.
In summary, among adolescents, the benefits of CHC in prevention of unplanned pregnancy generally outweigh the skeletal safety concerns . Clinicians should be aware of and able to counsel the adverse bone health effects of CHC. Aside from general recommendations to promote bone heath, DXA scans screening or monitoring of 25-hydroxyvitamin D level could be considered, especially in adolescents and young adults with long-term use of CHC.
Glucocorticoids
High-dose glucocorticoid treatment is often used in pediatric asthma exacerbation, rheumatologic diseases, autoimmune diseases, IBD, malignancy, and organ transplantation. The myriad adverse effects of glucocorticoids on growing skeleton are through several mechanisms . First, glucocorticoids decrease the estrogen and increase PTH level, thereby hasten bone loss by stimulating the osteoclast activity through RANKL/osteoprotegerin pathway. Second, glucocorticoids inhibit bone formation by inducing osteocyte and osteoblast apoptosis. Lastly, systemic glucocorticoid exposure can also lead to muscle wasting that contributes to further skeletal deficits. The net results are skeletal fragility and osteoporosis. A systematic review and meta-analysis of chronic glucocorticoid exposure and skeletal outcome in children and adolescents has shown a significantly lower LS aBMD when compared with healthy controls . The incidence of clinical fractures was reported to be 2%–33%, and vertebral fracture was highly prevalent (29%–45%). Studies using pQCT have made an interesting observation of differential effects of glucocorticoids on developing cortical and trabecular bone compartments. Reduced trabecular BMD and increased cortical BMD were noted following glucocorticoid exposure in children with various chronic diseases . It was hypothesized that glucocorticoid-induced low bone turnover leads to increased cortical porosity and prolonged secondary phase of bone mineralization.
The use of densitometric criteria for diagnosis of GIO in children and adolescents is challenging as small bone size from impaired linear growth is common with chronic glucocorticoid exposure. Furthermore, many fractures in GIO occur with a relatively normal aBMD . These findings highlight the limitation of DXA in predicting fracture risks in GIO and that other measures such as pQCT may be a preferred method for screening of adverse skeletal effects. Current guideline recommends clinical risk assessment at baseline (within 6 months of glucocorticoids initiation) and yearly in children and adolescents undergoing glucocorticoid therapy . The assessment includes detailed reviews of glucocorticoid use, other osteoporotic risk factors (e.g., malnutrition, thyroid diseases, and hypogonadism), and evaluation of clinical and asymptomatic fractures.
While bisphosphonate has been shown to be effective in reducing vertebral fracture risk and prevention of bone loss in adults treated with glucocorticoids , less is known about its safety and efficacy in children and adolescents with GIO. A randomized controlled trial in 22 children with chronic illness treated with prednisone found significant gains in LS vBMD in alendronate-treated group, but not in placebo group . An incidental fracture was noted in one patient treated with placebo but none in alendronate group. Our group has carried out a retrospective chart review of 29 patients with GIO and has found a trend of improvement in height-adjusted LS aBMD Z -score, although not clinically significant, at 1 year following IV bisphosphonate therapy (pamidronate or zoledronic acid) . Change in BMD at 1 year was not associated with fracture outcome. To date, there is insufficient evidence to support routine use of oral or IV bisphosphonate in children and adolescents undergoing chronic glucocorticoid therapy. Based on the current recommendation, bisphosphonate therapy and supplemental calcium and vitamin D could be considered in children and adolescents on chronic glucocorticoids ≥0.1 mg/kg/day for ≥3 months with clinically evident fragility fractures .
Gonadotropin-releasing hormone agonists
Gonadotropin-releasing hormone agonists (GnRH) agonists such as leuprolide have been used to slow linear growth and pubertal progression in children with central precocious puberty. The suppression of intrinsic sex steroid production by GnRH agonists gives rise to concern for the adverse effects on growing skeleton. A longitudinal study of GnRH agonists use in children with central precocious puberty or early puberty has found increased LS aBMD for age at baseline, followed by reduced LS and total body aBMD to normal range during treatment but lower than normal when corrected with bone age . Two years after cessation of GnRH agonists, there were absolute increases in BMD and BMAD in all children. In a randomized controlled trial, calcium supplementation (1 g daily) during GnRH agonists therapy has been found to prevent declined LS vBMD that occurred in a group that did not receive supplement . The effect of sex steroids interruption on skeletal health appeared to be transient as several studies have found normal peak bone mass in women received GnRH agonists in childhood .
Another emerging use of GnRH is in adolescents with gender dysphoria. Since the first development of pubertal suppression protocol by the Gender Identity Clinic at the VU University Medical Center in Amsterdam, The Netherlands, pubertal suppression has become a standard of care for management of gender dysphoria in adolescents. An Endocrine Society Practice Guideline published in 2017 recommends that Tanner stage 2 is the optimal time to initiate pubertal suppression, as early introduction of treatment has been shown to improve psychological and physical outcome associated with secondary sex characteristics that not aligned with individual gender identity . A pioneering study on bone health outcome following pubertal suppression in youth with gender dysphoria was carried out by Klink et al. . A decline in LS aBMD Z -score (compared with their natal sex) was observed following 1.3 and 1.5 years of pubertal suppression with triptorelin monotherapy in transwomen and transmen, respectively. Natal sex reference BMD values were used for aBMD Z -score calculation. Subsequently, one study of has demonstrated similar decline in aBMD Z -score and BTM, but BMAD at LS or femoral neck remained unchanged . Introduction of gender-affirming hormone (GAH) therapy with testosterone or estrogen is generally recommended at age 16 years if gender dysphoria persists . Interestingly, transgender woman individuals appeared to have low BMD prior to start of GAH when compared with males . Lower lean body mass and less physical activity have been postulated to be the contributing factors of this observation. The effects of GAH treatment on BMD have been evaluated in several studies. A meta-analysis has shown significant increases in LS aBMD at 12 and 24 months of GAH in transgender women but not in transgender men . Similar findings were observed in a 10-year follow-up study . One study using pQCT has found transgender women to have lower trabecular vBMD and aBMD at all skeletal sizes when compared with controlled male . Fracture outcomes have been examined in only one study . There was no incidental fracture during 1 year of GAH treatment. Current guidelines recommend a baseline bone age and DXA scans prior to initiation of GnRH agonists and serial DXA scans every 1–2 years during treatment . Youth with gender dysphoria who wish to undergo pubertal suppression should be counseled on potential adverse skeletal outcome and encouraged to optimize calcium and vitamin D intake and participate in regular physical activity to promote bone health. Bone health in children and adolescents with gender dysphoria has remained an area for further study, specifically assessment of fracture outcome and the effect of early introduction of GAH treatment prior to age 16 years, which has become more common practice nowadays.
Bariatric surgery
Bariatric surgery has become an emerging approach therapeutic approach of obesity in both adolescents and adults. Roux-en-Y gastric bypass (RYGB) was the most common procedure performed until recently that sleeve gastrectomy (SG) has become the most recommended approach for adolescents, given its excellent weight loss outcome and fewer operations . Data in adults have shown the negative impact of bariatric surgery on skeletal health, as evident by a significant decrease in BMD, increase in bone turnover, and increased fracture risks beyond initial period of weight loss . In a study utilizing QCT, bone microarchitecture continued to deteriorate at 5 years after procedure . The propose mechanisms of bone loss following bariatric surgery include decreased calcium and vitamin D absorption, decreased mechanical load to bone from weight loss, decreased circulating estrogen level and increased adiponectin level, and alteration of gut neurohormones . There are limited data on bone health outcome following bariatric surgery in adolescents. The only study to evaluate bone loss following bariatric surgery was a retrospective study of adolescents undergoing RYGB . Reduction in whole-body BMC was observed at 2 years following surgery and the loss correlated with weight loss. Although, BMD also declined, the aBMD Z -score remained appropriate for age. There has been a paucity of data on long-term bone health outcome and fracture risk following RYGB and SG in adolescents. At this time, nutritional monitoring and optimizing vitamin D and calcium intake have remained the mainstay recommendations for bone health monitoring.
37.3.3
Fractures in otherwise healthy children
Fractures are common health problem with a bimodal peak incidence in adolescence years and in the elderly. The incidence of fractures in children and adolescents has been reported between 12 and 36 per 1000 per year, with the peaks around the time of pubertal onset in boys and girls . This observation likely reflects the lag of bone mass accrual behind peak growth velocity . Distal radius is the most common site of fracture, followed by the phalanges and carpal bones . There are no established guidelines for evaluation and treatment of fractures in otherwise healthy children. In general, recurrent fractures or fractures in the absence of significant trauma warrant further evaluation to rule out primary bone diseases or secondary causes such as nutritional deficiencies or chronic disease. General measures to promote and protect bone health may be sufficient. Bisphosphonate use for treatment of recurrence fractures in otherwise healthy children has not been examined.
37.4
Treatment of osteoporosis
37.4.1
Optimizing bone health in children and adolescents
37.4.1.1
Calcium and vitamin D
Majority of total body calcium is found in bone, with over one-half laid down during adolescent years . A recent review of nine randomized controlled trials has found a small gain in bone mass by DXA or pQCT with oral calcium supplementation in most studies . There are conflicting data whether these gains persist after cessation of calcium supplementation . Findings from one cross-sectional suggested a protective effect of high dietary intake calcium on fracture (odds ratio 0.284) . Overall, there is insufficient evidence to recommend calcium intake more than dietary requirement for bone health benefits. The current recommendation for daily dietary calcium intake is 700 mg for children 1–3 years, 1000 mg for 4–8 years, 1300 mg for 9–18 years, and 1300 mg for pregnant or lactating women .
Vitamin D promotes intestinal calcium and phosphorus absorption and therefore plays an important role in calcium homeostasis and bone mineralization. Vitamin D deficiency is highly prevalent among children and adolescents . Common risk factors of vitamin D deficiency include darker skin color, obesity, winter season, and AED use. Vitamin D deficiency in adolescents has been linked to low BMD . However, a review of eight randomized controlled trials of vitamin D supplements has shown an improved hip BMC in female adolescents only .
37.4.1.2
Physical activity
The positive impact of weight-bearing exercise on a growing skeleton is well demonstrated. Randomized controlled trials of exercise (primarily jumping) in children and adolescents have shown better gains in bone mass and density . However, excessive exercise is linked to a twofold increased risk of fracture . To promote bone health in children and adolescents, current recommendations suggest to include at least 3 days of a week of bone-strengthening activity in the general recommendation of ≥60 min of daily physical activity. Further details on physical activity, exercise, and skeletal health are discussed in Chapter 22 , Physical activity, exercise and skeletal health.
37.4.1.3
Body weight and composition
Body weight and body composition are also important determinants of overall bone health. Greater detail is discussed in Chapter 44, Osteoporosis associated with eating disorders.
37.4.2
Bisphosphonate treatment
While minimizing risk factors associated with low BMD or secondary causes of osteoporosis are the mainstay therapeutic approaches for osteoporosis, pharmacologic therapy may be needed in children with primary osteoporosis or in those with secondary osteoporosis whose risk factors are unlikely to be mitigated. Bisphosphonates have long been used for treatment of OI on a compassionate care basis. This section discusses general information of bisphosphonate use in children and adolescents. Indications and outcomes of bisphosphonate use in specific disorders can be found in the previous sections of this chapter.
37.4.2.1
Effects of bisphosphonates on growing skeletons
Bisphosphonates are the pyrophosphate analogs with anti-resorptive property on bone. Bisphosphonate action on osteoclasts can be categorized into two mechanisms . First-generation (non-nitrogen-containing) bisphosphonates have a direct cytotoxicity to the osteoclasts, leading to osteoclast apoptosis. The second mechanism is involved in the interference osteoclast function by nitrogen-containing bisphosphonates. First use of bisphosphonates (oral pamidronate) was described in a child with OI in 1987 . The therapy has been used more broadly following an observational study of cyclic IV pamidronate in children with OI . Bone histomorphologic studies of children with OI, who had been treated with IV pamidronate for 2.4±0.6 years, showed a significant increase in cortical width, as a result of selective inhibition of bone resorption at the endocortical surfaces, while continuing bone formation on periosteal surfaces remained unaffected . These findings explain greater BMD and BMC increment in children when compared with adults.
As aforementioned, most data on the efficacy of bisphosphonates in treatment of pediatric osteoporosis are primarily from children with OI. A recent meta-analysis of 14 randomized controlled trials, 12 of which are studies in children, has shown significant improvement of BMD at various skeletal sites following oral or IV bisphosphonates in all studies . Although there was inconsistency in the fractures outcome, no studies reported an increased fracture rate following treatment. In longitudinal studies of IV pamidronate in children with OI, the majority of gains in BMD and cortical width occurred in the first 2 years of therapy and maintained for at least 2 years after cessation of treatment . However, discontinuation longer than 3 years created localized bone fragility, specifically at the junction between pamidronate treated (high-density) and pamidronate-naïve (low-density) bone . These observations have led to the recommendations to continue bisphosphonate therapy until linear growth is completed or near-completed in children and adolescents with primary osteoporosis. The most commonly prescribed IV bisphosphonate regimen is pamidronate 9 mg/kg/year every 3–4 months, more frequently in children <3 years due to higher bone turnover rate . Zoledronic acid has become increasingly used due to its shorter duration and less frequency of infusion. The commonly published regimen of zoledronic acid is 0.1 mg/kg/year every 6 months , with comparable efficacy profile to pamidronate . Many experts consider continuing treatment at reduced dose or less frequency once desired clinical outcomes (fracture, growth, bone pain, and BMD) have been achieved , albeit no standard protocol. Even though both oral and IV bisphosphonates have shown to be effective in improving BMD in previous studies and studies comparing overall fracture outcome between oral and IV bisphosphonates have shown conflicting results , oral bisphosphonate has no longer been a treatment of choice in OI. While IV bisphosphonates have been shown to improve vertebral body heights or vertebral reshaping , several studies of oral bisphosphonates have failed to do so . Furthermore, in a study of oral bisphosphonates (alendronate) of treatment of OI, bone histomorphometric changes, improvement in bone pain or functional outcome, were not observed following treatment . These findings could potentially be explained by very low bioavailability of oral bisphosphonates in children and adolescents with OI .
As previously discussed, less is known about the efficacy of bisphosphonates in treatment of secondary osteoporosis in children and adolescents. Moreover, there are no standard protocols for dose, frequency, and duration of treatment. Most published studies extrapolated treatment regimen from experience with OI . Several randomized controlled trials have examined the efficacy of oral and IV bisphosphonates , with an overall trend of improved BMD, but these studies were not adequately powered to draw the conclusion or measure fracture outcomes . Although short-term use of bisphosphonates in this population appeared to be relatively safe , at present, there is insufficient evidence to support routine use of oral or IV bisphosphonate in children and adolescents with disorders associated with secondary osteoporosis. Further studies are needed to determine the best therapeutic approach in this population.
Bisphosphonates have also been trialed in other pediatric bone and mineral disorders, such as fibrous dysplasia, avascular necrosis, primary bone tumor or bone metastasis, and hypercalcemia. Rationale and evidence of bisphosphonate therapy in these conditions are beyond the scope of this chapter.
37.4.2.2
Side effects and monitoring
Acute-phase reactions
Nitrogen-containing bisphosphonates, such as pamidronate and zoledronic acid, stimulate transient proinflammatory cytokines release that could bring about flu-like symptoms or the acute-phase reactions (APRs). APR may include low-grade fever, myalgia, bone pain, rash, and vomiting. APR is usually self-limited within 24–48 hours but can last up to several days . Symptomatic treatment with antipyretic or antiemetic medications and fluid hydration is usually sufficient. It has been observed that APR is more common in bisphosphonate naïve patients. In a retrospective study of over 900 IV bisphosphonate infusions for treatment of pediatric osteoporosis, APR was reported in 27% following the first infusion, compared with 5% in subsequent infusions . In this study, APR was more common in patients with secondary osteoporosis and associated with use of zoledronic acid. Underlying illness, such as chronic inflammation, may put children and adolescents with secondary osteoporosis to be more prone to APR. Many practices have used half of the recommended dose for an initial infusion with or without prophylactic antipyretic medications to lessen the possibility of developing APR.
Electrolyte abnormalities
Hypocalcemia and hypophosphatemia are also common side effects of IV bisphosphonates. The inhibition of osteoclast activity is likely responsible for hypocalcemia, although severe hypocalcemia is not common . Half-dose IV bisphosphonates for the first infusion followed by a short course of calcitriol and/or calcium supplementations may be considered to reduce severity of hypocalcemia . Hypophosphatemia is also common but rarely requires intervention . Prior to first dose of IV bisophosphonates, it is generally recommended to assess complete blood count, serum electrolytes (including calcium, phosphate, and magnesium), and liver profiles . Vitamin D deficiency should also be screened and treated, if present. There is variability of approaches to monitor serum electrolytes following IV bisphosphonates (e.g., 24, 48, 72 hours, or 7 days).
Delayed osteotomy healing
Due to the suppression of bone turnover, there has been a concern that bisphosphonates may interfere bone healing process following osteotomy with intramedullary rod placement. Although one study demonstrated delayed osteotomy healing in children with OI receiving bisphosphonates , a more recent study has reported less frequency of delayed healing with new surgical approach and zoledronic acid use . Despite no study on the best interval between bisphosphonates administration and surgery, many experts recommend the 4–8 weeks interval to allow optimal bone healing.
Atypical femur fracture
AFFs at subtrochanteric and diaphyseal region have been reported in adults with or without exposures to bisphosphonates . The characteristics of AFF are non-comminuted, transverse fractures originating at the lateral cortex with localized periosteal or endosteal thickening that occur with minimal trauma. Adults with long-term bisphosphonate use are at increased risk of AFF, estimated 100 per 100,000 person-years . AFF is less common in children and adolescents and primarily reported in OI , although recent study has found no correlation between AFF with bisphosphonate use, but rather with the severity of OI . To date, there are two reports of AFF in children without primary collagen defects treated with bisphosphonates .
Osteonecrosis of jaw
Osteonecrosis of jaw (ONJ) is another rare complication that has been linked to long-term bisphosphonate therapy in adults. Although ONJ has not been reported in children, many practices recommend preventive approach with regular dental evaluation every 6–12 months during bisphosphonate therapy .
In summary, despite apparent benefits of bisphosphonate therapy in children and adolescents with bone fragility, especially primary osteoporosis, risk and benefit of treatment should be carefully reviewed and caution is still needed when considering starting therapy.
37.4.3
Novel hormone therapy
37.4.3.1
Parathyroid hormone analogs
Many secondary osteoporosis conditions in children and adolescents, such as DMD and GIO, are characterized by decreased bone turnover and bone formation. While there have been evidence of potential benefits from bisphosphonate use in these conditions, anabolic agents have remained an appealing therapeutic approach as they target the underlying pathophysiology of these conditions by promoting new bone formation.
PTH (teriparatide, PTH 1–34) increases bone formation when administered intermittently. Abaloparatide (PTH-related protein analog) activates PTH 1 receptor and results in net anabolic effects on bone. Teriparatide and abaloparatide have been shown to not only improve BMD but also reduce risks for vertebral and nonvertebral fractures in postmenopausal women and are approved as first-line pharmacologic treatment for severe osteoporosis in this population . In a comparison study, abaloparatide was associated with greater proportion of patients who experienced increase in BMD and lower incidence of hypercalcemia when compared with teriparatide . Rapid bone loss has been observed following cessation of PTH analogs therapy, therefore, continuation of anti-resorptive is recommended. In adults with GIO, teriparatide has shown superior effect on BMD gain when compared with alendronate . There is paucity of studies of PTH analogs for treatment of osteoporosis in children with open epiphyses, given the FDA black box warning against its use in children based on increased incidence of osteosarcoma in rats, albeit the dose used in that study was much higher that what typically used in human . A case report of teriparatide in a 20-year-old man with DMD who suffered multiple vertebral fractures has shown improvement of LS aBMD at 12 and 18 months of treatment, improved quality of life, stable vertebral deformities, and no significant safety concerns . Teriparatide use was reported in another 19-year-old patient with OPPG who failed to show further improvement of aBMD following 6 years of cyclic pamidronate infusions . A 1-year course of teriparatide, started 1 year following discontinuation of bisphosphonate, has shown to improve LS and hip aBMD with no adverse effects. The clinical utility of abaloparatide in children and adolescents has not been studied.
37.4.3.2
Denosumab
Denosumab has an anti-resorptive property by inhibiting RANKL, thereby decreasing the osteoclast activity. It has been shown to effectively reduce vertebral, hip, and non-vertebral fractures and is approved as first-line pharmacologic agent for postmenopausal osteoporosis . There has been an emerging interest for its use for treatment of osteoporosis and other skeletal disorders in children and adolescents. Denosumab use in children and adolescents with OI has shown modest increase in BMD in some but not all studies . An improvement of LS aBMD was also observed in a case report of a 13-year-old boy with DMD treated for osteoporosis . The side effects reported in these studies include hypocalcemia and rebound hypercalcemia given its short duration of action. Larger perspective studies to determine optimal dose and duration of denosumab, and studies comparing with conventional therapy with bisphosphonate for treatment of osteoporosis in children and adolescents are warranted.
37.4.3.3
Romosozumab
Romosozumab is an anti-sclerostin antibody. By blocking sclerostin action, romosozumab promotes bone formation and decreases bone resorption . It has been shown to improve BMD and reduce vertebral and nonvertebral fractures in adults and is approved for treatment of adult osteoporosis in many countries, including in the United States . At the present time, data on of romosozumab in children and adolescents are not yet available.
37.5
Summary and future directions
Although there have been advances in understanding of the genetics of primary osteoporosis, and the pathophysiology and natural history of secondary osteoporosis in childhood and adolescence, additional research related to both the diagnosis and treatment of pediatric osteoporosis is needed. The recognition of limitations of bone densitometry in growing skeleton has moved BMD away from a sole measure in the diagnosis or management of pediatric and adolescent osteoporosis. Future longitudinal observational studies or controlled trials focusing on bone quality and fracture outcomes are needed to solve many unanswered questions about primary forms of osteoporosis and the effects of chronic diseases on growing skeleton in children and adolescents. Even though there are growing evidence supporting use of bisphosphonates to prevent fragility fractures in certain children and adolescents, the consensus on drug of choice, optimal dose and duration of treatment, and the protocol for monitoring of the safety and efficacy of treatment are still lacking. Novel therapeutic approaches such as PTH analogs and anti-sclerostin antibody have shown promising results in adults and merit further study in the pediatric population.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








