Stage
Clinical characteristics
Radiographic findings
Stage 0
No evidence of necrotic bone, but presence of nonspecific signs or symptoms
• Odontalgia not explained by an odontogenic cause
• Dull, aching bone pain in the body of the mandible, which may radiate to the temporomandibular joint region
• Sinus pain, which may be associated with inflammation and thickening of the maxillary sinus wall
• Altered neurosensory function
• Loosening of teeth not explained by chronic periodontal disease
• Periapical/periodontal fistula or sinus tract that is not associated with pulp necrosis due to caries
• Gingival swelling/inflammation with or without crevicular exudate (spontaneous or on palpation)
• Persistence of unremodeled extraction socket
• Prominent osteosclerosis in a jawbone region with changes to trabecular pattern
• Alveolar bone loss or resorption not attributable to chronic periodontal disease
• Thickening/obscuring of the periodontal ligament (thickening of the lamina dura and decreased size of the periodontal ligament space)
• Inferior alveolar canal narrowing
Stage 1
• Exposed and necrotic jawbone evident in the oral cavity
• Asymptomatic
• No soft tissue infection or purulence
• Lytic bone lesion
• With or without evidence of sequestrum
• No cortical perforation or periosteal bone formation
Stage 2
• Exposed and necrotic jawbone evident in the oral cavity
• Inflammatory signs and symptoms
• Infection/purulence
• Lytic bone lesion
• Sequestrum centrally
• Displacement of adjacent anatomic structures such as teeth which may have thickened lamina dura
• May have cortical involvement
Stage 3
• Exposed and necrotic bone evident in the oral cavity
• Inflammatory signs and symptoms
• Infection/purulence
• One or more of the following:
– Sequestrum extending beyond the alveolar bone area
– Pathologic fracture
– Extraoral fistula
– Oroantral/oronasal communication
– Osteolysis extending to the inferior border of the mandible or the antrum of the sinus or zygoma in the maxilla
• Extensive lytic bone lesion
• Sequestrum
• There may be evidence of cortical expansion, thinning, erosion and perforation, and/or periosteal bone formation
• Displacement of adjacent anatomic structures such as teeth, the inferior alveolar nerve canal, or the maxillary sinus
• Tooth involvement may appear as thickened lamina dura
• Pathologic fracture may be observed
Stage 0 cases show no clinical evidence of exposed necrotic bone, but nonspecific clinical or radiographic findings as described in Table 12.1. Figure 12.1 –>shows an example of stage 0 ARONJ in a patient taking Dmab for osteoporosis . Stage 0 cases can be the most challenging to diagnose clinically given the nonspecific signs and symptoms and the fact that some cases may represent another disease process and not ARONJ. Only with hindsight and disease progression, in the presence or absence of therapy, could it be deemed that indeed a case presumed to be stage 0 actually represents stage 0 ARONJ. For example, one could argue that the patient in Fig. 12.1 has a periodontal abscess only and should be treated as such; subsequently, if there is lesion resolution with appropriate treatment for a periodontal abscess, it would be impossible to definitively diagnose the case as ARONJ unless overt bone exposure were to occur at some point in the future despite the treatment. This is just one example of many with respect to nonspecific presentations of ARONJ and the potentially broader differential diagnosis as experienced by clinicians in evaluating challenging stage 0 cases of disease.
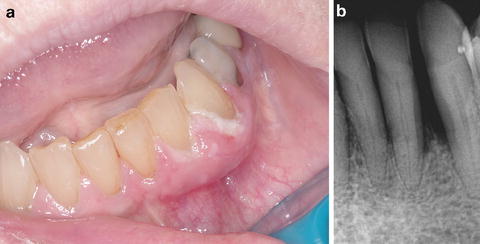

Fig. 12.1
(a) Seventy-six-year-old Caucasian female on Dmab therapy for osteoporosis for 2 years duration, presenting with loose teeth and swelling of the left mandibular anterior gingiva and purulent crevicular exudate from the periodontium, but no overt evidence of exposed bone. (b) Periapical dental X-ray of the area reveals ill-defined lytic change and bone loss around affected teeth with widening of the lamina dura space (stage 0 ARONJ)
The clinical features of stage 1 cases include the presence of exposed necrotic bone, but no evidence of soft tissue infection or purulence. Figure 12.2 shows an example of stage 1 ARONJ in a patient taking oral ibandronate for osteoporosis. Stage 2 is characterized by exposed necrotic bone that is associated with signs of infection (e.g., pain, erythema, and/or purulence). Figure 12.3 shows an example of stage 2 ARONJ in a patient taking oral alendronate for osteoporosis. Stage 3 cases exhibit more extensive necrotic bone and infection which extend beyond the alveolar region or osteolysis that extends to the inferior border of the mandible or the sinus floor, amenable to pathologic fracture, extraoral fistula, and oroantral or oronasal communication [16]. Figure 12.4 shows a patient with cellulitis involving the right mandible and face, from spread of ARONJ infection.
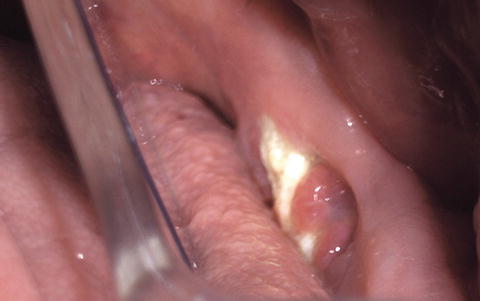
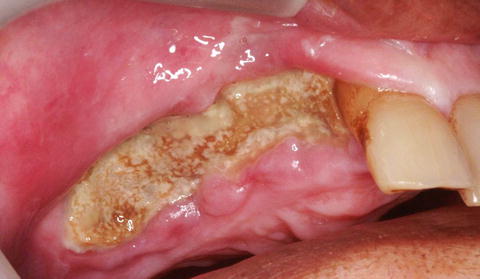
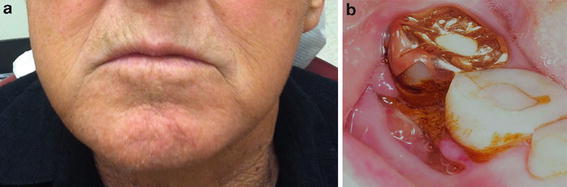

Fig. 12.2
Seventy-one-year-old Caucasian female with a 4-year history of oral ibandronate use for osteoporosis, presenting with an asymptomatic area of exposed bone and associated granulation tissue involving the lingual surface of the left posterior hemimandible (stage 1 ARONJ)

Fig. 12.3
Seventy-eight-year-old Caucasian male with a 6-year history of oral alendronate use for osteoporosis, presenting with a painful area of exposed and infected bone involving the right hemimaxilla (stage 2 ARONJ)

Fig. 12.4
(a) 69-year-old male with diffuse right facial swelling, pain, and cutaneous sinus tract formation (see Fig. 12.9) consistent with a stage 3 ARONJ. (b) Intraoral photograph of the same patient shows the source of the infection from the exposed bone and inflamed gingiva on the buccal aspect of the posterior right mandibular molars
To address NE variants of ARONJ, which can resemble advanced stages of disease clinically, the AAOMS staging system has been adapted to include the addition of NE variants of ARONJ for each stage [13]. Aside from forming the basis for diagnosis guidelines, staging systems for ARONJ direct appropriate treatment for each stage. However, there is currently no clear consensus on appropriate therapeutics.
Pathogenesis
The pathogenesis of ARONJ is multifactorial and may be different for n-BP cases as compared to Dmab cases; however, similarities or common pathways may also exist. Much more investigation and literature is available on the pathogenesis of n-BP ARONJ and virtually little to none on Dmab ARONJ . Alterations in bone remodeling, antiangiogenic effects, matrix necrosis, tissue toxicity, immunomodulation, and infection have been proposed to play roles in ARONJ pathogenesis. n-BP compounds are known to deposit in the bone compartment, where they inhibit osteoclast activation and promote osteoclast apoptosis by several mechanisms, including inhibition of protein prenylation and blockade of mevalonate metabolism [17]. They also have antiangiogenic properties [18] and inhibit matrix metalloproteinases [19] and in vitro proliferation of oral keratinocytes [20]. The toxic or inhibitory effect on oral epithelial growth by n-BP drugs may explain the delayed wound healing seen in ARONJ cases.
Knowledge of the pharmacology (pharmacokinetics and pharmacodynamics) of n-BP drugs is essential to understanding most cases of ARONJ disease and pathogenesis. Once resident in bone, n-BP compounds are usually eliminated during bone resorption—a process that can take years [21]. This results in a long half-life in bone, for example, up to 10 years for alendronate [21]. Orally administered n-BP drugs have a very low bioavailability (0.3–6 %), whereas intravenous administration provides much higher bioavailability, allowing for greater drug accumulation in the skeleton; for example, 50 % of an intravenous-administered therapeutic dose of the n-BP pamidronate concentrates selectively in the skeletal compartment from the plasma, while the rest is excreted unchanged in the urine [22]. These differences in oral versus intravenous dosing and bone bioavailability may explain the greater frequency of ARONJ in oncologic versus rheumatologic dosing. Further, there is significant interpatient variability in n-BP pharmacokinetics and biodistribution , but little intrapatient variability [22]. Significant differences also exist in the potency and binding affinities of various n-BP drugs to bone. These differences in the pharmacokinetics and pharmacodynamics of the various n-BP drugs can impact bone binding and release of drug, the observed clinical differences in potency and duration of effect, and complications like ARONJ.
Not only does bone acts as a reservo ir for n-BP, but the drug can also be released during bone resorption or trauma and surgery to bone . The concentration of active n-BP drug released into the surrounding jawbone environment by osteoclastic resorption is approximately one-half that of the concentration of drug in bone. In vitro thresholds for toxicity to cells in the local area essential for covering exposed alveolar bone are known. For example, oral mucosal wound healing stops at 0.1 mM of pamidronate [20]. Similar studies with osteoblasts, endothelial cells, fibroblasts, T-cells, and macrophages demonstrate a threshold-dependent toxicity to concentrations of n-BP drugs in vitro [20]. Therefore, after a tooth extraction or oral surgical procedures to bone, which is when most cases of ONJ occur, bound n-BP from both superficial and deep layers of bone is released into the local milieu where it can inhibit oral wound healing in addition to other effects which we discuss shortly and which have been validated in vivo.
Investigators have questioned why the jawbones are predominantly affected by osteonecrosis when n-BP drugs disseminate to all bones of the body. Accordingly, one popular hypothesis for ARONJ pathogenesis is that there is higher bone remodeling in the jawbones as compared to other skeletal sites and greater remodeling in the mandible as compared to the maxilla. Masticatory load and mechanics could potentially result in greater accumulation of n-BP compounds in the mandible versus the maxilla or other osseous sites such as the femur. However, a recent study evaluating bone scintigraphy scans of cancer patients prior to and throughout the course of n-BP therapy revealed a similar bone turnover in the mandible and the femur and a significantly lower bone turnover in the mandible as compared with the maxilla [23]. All investigated bone regions showed no significant changes throughout n-BP administration, and bone remodeling in the jawbones was not overly suppressed by ART [23]. The finding that the mandible has a significantly lower bone turnover than the maxilla, and the fact that most ARONJ cases occur in the mandible, led the authors to suggest that the aforementioned popular hypothesis for ARONJ pathogenesis is not plausible. Additionally, uptake of n-BP has been shown to be higher in the axial skeleton compared with the appendicular skeleton or craniofacial bones; teeth and jawbones show no exceptional differences in drug uptake compared with other hard tissues [24]. Therefore, common notions with respect to pathogenesis—that there is preferential uptake of n-BP drug to jawbones and greater remodeling in the jawbones as compared to other bones in the body—may be unfounded.
There is, however, mounting ev idence that the jawbones are unique from other bones in the body mainly in their susceptibility to ARONJ because of the presence of bacteria in the mouth and saliva which have ready access to jawbone [25, 26]. Histopathologic findings indicate that even the healthy edentulous jaw can contain regions of nonviable bone and microbial biofilm formation for more than one year after tooth extraction and normal mucosal healing [27]. Regions of nonviable bone and subclinical infection may contribute to the development of untoward clinical events such as ARONJ. Recent microbiologic findings reveal a characteristic pathogenic profile of organisms in patients with ARONJ compared to patients without disease [28]. This pathogenic profile is dominated by a few phyla, mainly Proteobacteria, Firmicutes, and Actinobacteria and predominantly facultative anaerobes. The same phyla have been shown to dominate symptomatic dental periradicular infections as analyzed by 454-pyrosequencing [29]. These pathogens reside in saliva, infected teeth, and periradicular lesions and can easily gain access to jawbone, especially after invasive dental procedures that expose bone, which is when most cases of ARONJ occur [30]. All studies to date examining ARONJ-affected bone histopathologically report microbial colonization [31].
Recent investigations have also sh own that n-BP drugs not only inhibit oral wound healing and bone healing but also facilitate bacterial colonization on bony surfaces where both drug and biofilm bacteria co-localize [32]. Therefore, the clinical problem of ARONJ is essentially a biofilm-mediated osteomyelitis of the jawbones secondary to poor wound healing from toxic accumulation of n-BP [33]. The common role of infection in ARONJ pathogenesis explains the current antimicrobial and surgical approaches to ARONJ therapy, the finding of ONJ with other classes of drugs not related to ART, and the fact that patients are at risk for disease years after discontinuation of n-BP given the long bioavailability of n-BP and of pathogens in jawbone.
The development of animal models has provided additional insights into ARONJ pathogenesis. For example, it has been shown that osteoclasts at different anatomic sites internalize n-BP differentially, but differential uptake does not correlate directly with osteoclastogenesis or osteoclast precursor sensitivity to drug [34]. Propensity to n-BP is site-specific and varies in the jawbones as compared to the femur; jawbone osteoclasts are more susceptible to inhibition of prenylation. Further, n-BP accumulation in bone has been shown, contrary to popular belief, to be an e quilibrium-dependent drug–crystalline bone mineral interaction which more accurately explains n-BP biodistribution and pharmacokinetics and may be more relevant to ARONJ pathogenesis [35]. These findings reveal that although the jawbones do not take up more n-BP than other skeletal sites, jawbone osteoclasts may be more sensitive to the effects of n-BP than osteoclasts at other sites. Recent animal studies have also revealed a role for immune dysregulation in ARONJ pathogenesis [36]. Additionally, animal studies provide the opportunity to test novel therapeutics such as molecular and stem cell-based approaches to ARONJ treatment. However, extrapolating data from animal studies directly to humans is problematic as there are considerable differences in bone remodeling, pharmacokinetics, microbiome, immune responses, and oral function. Further studies in humans and higher levels of evidence are necessary before direct translation to humans.
Finally, the “-omics” revolution and advanced molecular methodologies have provided knowledge into ARONJ pathogenesis through evaluation of the salivary proteome [37], the pharmacogenome via genome-wide association studies [38], the microbial metagenome or microbiome [39], and single nucleotide polymorphisms [40, 41] associated with disease. This line of investigation has the potential to reveal differences or unique signatures in patients affected with ARONJ as compared to those without disease, allowing for development of potential clinical biomarkers in the future. Prospective well-controlled studies using a transdisciplinary team of clinicians and basic scientists, with bioinformatic and computation biology analyses and a hypothesis-driven approach, will be essential for advancement in understanding ARONJ pathogenesis and risk, as well as for biomarker dev elopment and validation.
Risk
To date, most ARONJ literature represents lower levels of evidence and a weak strength of evidence for direct translation and application to clinical understanding. These publications include editorials, expert opinions, in vitro models, animal models, case reports, case series, and retrospective studies. Few prospective well-controlled human studies exist, which has hindered accurate disease characterization and risk assessment. The available literature that represents relatively higher levels of evidence, such as systematic reviews and case–control, cohort, and controlled longitudinal studies, provides insight into risk factors for ARONJ. Table 12.2 lists potential risk factors that have been reported to be associated with ONJ in general [6, 9, 42–45]. Further studies are needed to more accurately address risk and risk assessment for ARONJ specifically, and many currently proposed risk factors require validation. Additionally, risk factors differ for rheumatologic versus oncologic patients receiving ART.
Table 12.2
Potential risk factors for ONJ
• Dental risk factors (periodontal or periapical disease, oral trauma, extractions, implants) |
• Dose and duration of ART therapy |
• Cancer |
• Osteoporosis |
• Corticosteroids |
• Chemotherapy |
• Antiangiogenics |
• Immunotherapy |
• Female sex/estrogen therapy |
• Advanced age |
• Ethnicity (Asian race highest risk) |
• Smoking |
• Anemia |
• Diabetes |
• Arthritis |
• Hypothyroidism |
• Storage diseases |
• Systemic lupus erythematosus |
• Hypertension |
• Hemodialysis |
• Blood dyscrasias |
• Vascular disorders |
• Chemical exposure |
• Herpes zoster |
• Alcohol abuse |
• Coagulation abnormalities |
• Gaucher disease |
• Human immunodeficiency virus infection |
• Hyperlipidemia and embolic fat |
At currently available levels of evidence, it is difficult to stratify risk (e.g., low to high), and it is more appropriate to identify patients that are potentially at risk. Risk assessment must involve consideration of medical, dental, and medication history, in addition to other clinical parameters. Risk assessment aids in identifying patients unaffected but susceptible to ARONJ, allowing preventative and dental prophylactic measures to be instituted when necessary [46]. Risk reduction efforts may involve restorative or endodontic dentistry to avoid extractions or invasive dental procedures when possible or the im plementation of antibiotic prophylactic protocols prior to and after necessary invasive dental procedures, which has been shown to significantly reduce risk and ARONJ incidence [47].
More recently, the application of pharmacometric and bioinformatic analytical tools and predictive modeling to patients with ARONJ has enabled the quantification of drug levels in the bony compartment, allowing determinations of drug accumulation to potentially toxic levels for induction of ARONJ [48]. Pharmacometrics is a burgeoning field that has enabled personalized medicine and individualized pharmacotherapy and has provided significant clinical insight into drug-associated conditions [49]. In the future, the application of this approach clinically will give practitioners a quantitative pharmacokinetic method for determining drug accumulation in bone without the need for compartmental measurements or invasive procedures, serving as a powerful risk assessment tool to guide clinical decision-making. Pharmacometric modeling of accumulation of n-BP in b one has validated that ONJ is predictable in dental surgery patients who have acquired sufficient concentrations of drug to liberate aqueous solutions concentrated above the 0.1 mM in vitro threshold required to impair cell migration and completely inhibit intracellular production of isoprenoid lipids. These population-based pharmacometric tools have also identified ethnic predispositions to ARONJ and indicate that Asians have the highest risk due to anthropometric and potentially pharmacogenomic parameters [48], which has been partially validated in large epidemiologic studies [45]. Finally, biomarkers associated with bone physiology (e.g., CTX, NTX, BAP, OC, DPD, PTH) have been investigated for their utility in ARONJ risk assessment, but there is currently insufficient prospective evidence for risk prediction of ARONJ based on biomarkers, and additional research and validation are n ecessary [50].
Clinical Features
Patients with ARONJ can be asymptomatic or may have a variety of signs and symptoms. Some patients with early stage ARONJ, in both exposed and NE variants, may have no associated symptoms and may be unaware of having disease until diagnosed by a health-care provider. In NE cases at early stage, diagnosis may be delayed until overt signs or symptoms become evident with disease progression. Early symptomatology includes discomfort and an abnormal feeling in the mouth. Most ARONJ cases are associated with precedent oral trauma or infection , such as tooth extraction or an abscess, with delayed wound healing [51]. Therefore, dental consultation and evaluation is prudent prior to the initiation of, or especially during, ART.
A common presentation for ARONJ is a nonhealing ulcer or extraction socket (Fig. 12.5). The mandible is affected more often than the maxilla. When exposed bone or sequestrum is present, patients may feel the ulcerative lesion with their tongue as a rough hard area (the sequestrum) with surrounding irregular soft tissue. Patients with tori or exostoses are particularly susceptible to ARONJ because the overlying mucosa is normally attenuated and prone to trauma and injury. Figure 12.6 shows a typical lesion of ARONJ involving mandibular tori. Epithelialization over tori even at early stage disease without signs of infection is difficult to achieve naturally and may require some surgical intervention.
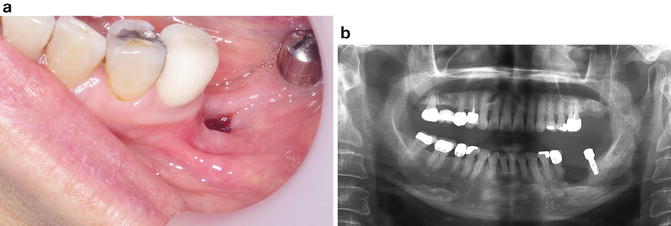
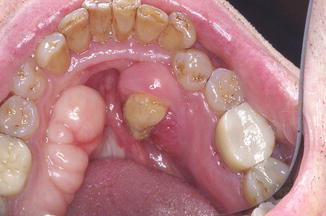

Fig. 12.5
(a) 57-year-old female with a history of several years of oral ART presenting with a nonhealing extraction site 6 months postoperatively in the left posterior mandible anterior to a dental implant. (b) Panoramic radiograph of the same patient shows a relatively well-defined saucerized radiolucent lesion in the region with suggestion of central sequestrum formation

Fig. 12.6
Sixty-four-year-old male with a painless area of exposed bone and stage 1 ARONJ involving mandibular tori
Inflammatory signs and symptoms such as pain and erythema can be a common finding with ARONJ, usually with progression of disease past stage 1. Inflammation of soft tissues surrounding affected bone may result in the appearance of swollen and/or bleeding gingival tissues as shown in Fig. 12.7. Loosening of teeth may occur due to periodontal involvement of ARONJ. Purulence may be an important finding with disease progression and may be accompanied by malodor or bad breath. Culture of pus from ARONJ lesions with antibiotic sensitivity testing may help guide antimicrobial therapeutics, and notoriously pathogenic oral bacteria are usually cultured. Exposed sequestrum may be sharp, causing irritation and traumatic ulcers of adjacent mucosa such as the tongue or buccal mucosa. Figure 12.8 shows a ventrolateral tongue ulcer secondary to trauma from exposed sharp sequestrum in ARONJ. Patients with prosthodontic or dental appliances may complain of ill-fitting prostheses due to inflammatory changes in underlying affected tissues, and this can be an early symptom of ARONJ. With disease progression, patients may complain of difficulty eating or dysphagia, and weight loss can be observed in such cases. Fever is only seen in more severe cases or advanced-stag e disease. With fever or active purulence from lesions, systemic hematology lab values may show increased leukocyte counts, particularly lymphocytosis or neutrophilia with or without evidence of neutrophil bands.
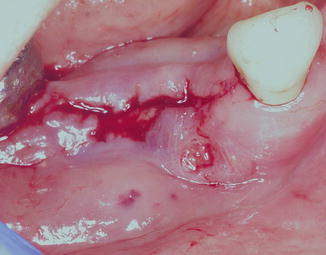
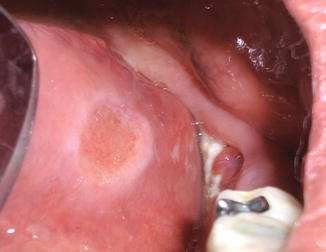

Fig. 12.7
Fifty-nine-yea r-old female with a stage 2 ARONJ lesion that bleeds spontaneously and has frequent episodes of exudate coming from the lesion, with concomitant malodor. The gingival swelling and bleeding can make it difficult to visualize the exposed subjacent bone; therefore, gentle saline irrigation may be required in order to do so clinically

Fig. 12.8
The same pati ent as shown in Fig. 12.2 with an ulcer of the left ventrolateral tongue due to trauma from the adjacent area of exposed bone. In cases like this, curettage, filing, or debridement of the bone should allow for tongue tissue healing within a few weeks. If healing does not occur after addressing the traumatic etiology, biopsy of the tongue ulcer may be warranted to rule other conditions in the differential diagnosis of ventrolateral tongue ulcers, such as squamous cell carcinoma
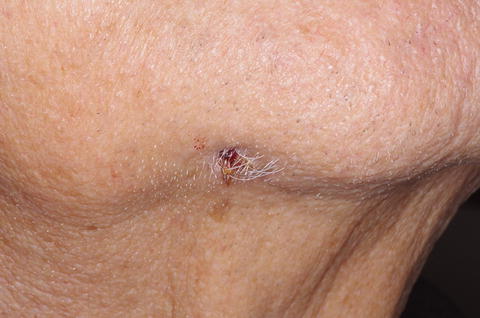
When ARONJ occurs in the mandible and there is inferior alveolar nerve involvement, patients may have sensory complaints such as numbness or tingling in the lower jaw and lip on the affected side of the face. Sensory complaints may represent paresthesia, dysesthesia, allodynia, hypesthesia, or anesthesia. In advanced stages of disease, pathologic fracture of the jawbone, oroantral or oronasal communication, or sinus tract formation to the skin of the face can occur [52]. Figure 12.9 shows a cutaneous sinus tract , which developed from an advanced ARONJ that was associated with facial cellulitis. Cellulitis can be seen in severe cases with infection dissemination through facial spaces; maxillary phlegmon may involve canine or buccal spaces, and mandibular phlegmon may involve submental, sublingual, and submandibular spaces [53]. Further direct or lymphatic spread may occur to secondary spaces such as pterygomandibular, lateropharyngeal, masseteric, and pterygomaxillary spaces.