Fig. 3.1
PTH causes accrual of new bone by mainly remodeling-based “overfilling” of resorption pits (lower panel), but a certain degree of modeling-based accrual of bone on surfaces without preceding resorption is taking place (upper panel)
While remodeling balance in moderate primary hyperparathyroidism is neutral [15], intermittent administration of PTH causes overfilling of resorption lacunae (Fig. 3.1). Based on the histological studies conducted so far, the remodeling-based bone accretion seems to supersede modeling-based accretion [16, 17].
Intermittent administration of PTH also induces increases in IGFs and other anabolic growth factors (BMPs, cbfa1), and the increased IGF-1 levels have been implicated in PTH-induced stimulation of hematopoiesis [18]. A similar role has also been proposed for the newly discovered nuclear matrix protein 4/cas interacting zinc finger protein (Nmp4/CIZ) [19]. Immunohistochemical studies on human bone biopsies have demonstrated increased levels of IGF-2 in the bone matrix after PTH treatment [5, 17].
Recent data suggest that bone formation induced by osteocytic PTH receptor signaling on the periosteal surface depends on Wnt signaling but not on resorption, while bone formation on the endocortical surface results from a combination of Wnt-driven increased osteoblast number and modulation of osteoclast–osteoblast cross talk [20].
Bone histomorphometry studies after therapy with PTH reveal increased remodeling as reflected in increased osteoid synthesis, mineralization of bone surfaces, and increased osteoblastic vigor as reflected by an increased distance between double tetracycline labels [21]. The increased bone formation eventually results in pronounced improvements in cancellous and cortical bone. Cancellous bone volume increases by an average of 35 %, and trabecular connectivity, which is reduced in osteoporosis, is improving as well. Also, trabecular number increases, and trabecular morphology returns to a more platelike appearance as seen in younger individuals and different from the more rodlike shape of osteoporotic bone. Cortical thickness and cortical cross-sectional area also increase, which is an effect not seen with antiresorptive treatments [22]. Quantitative CT (QCT) has also corroborated these improvements in cancellous and cortical bone structure in vivo [23, 24].
Bone matrix formed during PTH treatment exhibits less mineralization and less collagen cross-links typical of more immature bone [25]. Albeit the impact of microcrack accumulation after antiresorptive treatment on bone quality still remains obscure, PTH treatment has been shown to reduce microcracks in patients pretreated with alendronate [26].
Effects of PTH at the Organ Level
In humans, intermittent administration of teriparatide leads to a rapid increase in bone formation markers followed sometime (usually about 2–3 months later) by increases in bone resorption markers. This sequence of events has led to the concept of the “anabolic window,” a period of time when the actions of PTH are maximally anabolic. Bone formation markers peak at 6 months, and then they gradually return toward baseline over a period of 2–3 years with some interindividual variation (Fig. 3.2) [27]. The reasons behind this apparent refractory response of human bone is largely still unknown, but a treatment period of more than 14 months seems important to maximize anti-fracture efficacy [1].
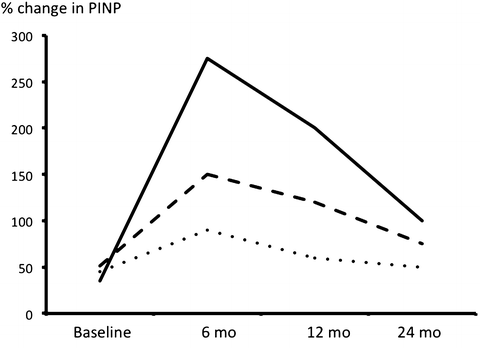

Fig. 3.2
Changes in a marker of bone formation (PINP) after initiation of therapy with PTH(1–34) in 3 patients. Note the peak at 6 months
Quantitative radionuclide imaging studies using technetium-99m methylene diphosphonate [(99m)Tc-MDP] have demonstrated accumulation of isotope in the calvarium, mandible, spine, pelvis, and upper and lower extremities [28]. Median increases from baseline in bone turnover as assessed with this technique were 22 % at 3 months and 34 % at 18 months. All subregions exhibited increased an accumulation at both time points except for the pelvis, and these increases at organ level revealed significant positive correlations to markers of bone formation (BAP and PINP). Another study from the same group using PET technology demonstrated that the increase in bone turnover was more pronounced in cortical than trabecular bone [29].
Another effect seen after treatment with PTH, which has not been reported for antiresorptive agents, is an increase in bony dimensions. Peripheral QC measurements and analyses of hip DXA scans have demonstrated a dose-dependent increase in cortical thickness, bone perimeter, and bending strength in the forearm and hip after PTH treatment [30, 31]. Other studies were, however, unable to corroborate such increases [24]. For vertebral bodies, two studies have reported increases in vertebral cross-sectional area (CSA), which would potentially lower fracture risk further, but the estimates differ widely ranging between 4.8 % [32] and 0.7 % [33].
The stimulation of bone formation causes a dose-dependent increase in BMD amounting to 6–9 % over placebo in the spine and 3–6 % at the hip with 20 and 40 μg daily dosing after a median treatment period of 21 months [34]. At certain sites rich in cortical bone, such as the distal 1/3 of the radius, PTH typically does not increase bone density. In fact, there may be a small decline in BMD. Early BMD assessment at the hip usually reveals a decrease too, which then gradually reverses into an increase. This is mainly caused by an increase in cortical porosity accompanying the increase in overall bone turnover and to a lesser extent the formation of new, less mineralized bone together with dimensional changes. This notion is further corroborated by the fact that the maximal decrease in porosity is seen after 6 month treatment duration where the activation of bone remodeling is at its highest [21]. The transient reduction of BMD does not translate into decreased bone strength, however, because the increased porosity occurs only in the inner one third of bone, where the mechanical effect is minimal. Moreover, the changes in bone geometry and microarchitecture (increased cortical thickness) more than compensate for any potential adverse effects of increased cortical porosity on bone strength [35].
Effects on Vertebral Strength
Using FE modeling on QCT imaging from patients treated with either PTH(1–34) or alendronate, Keaveny et al. [36] reported that both treatments induced positive effects on vertebral strength characteristics (Fig. 3.3). At least 75 % of the patients in each treatment group had increased strength of the vertebra at 6 months compared with baseline. Patients in both treatment groups had increased average volumetric density and increased strength in the trabecular bone, but the median percentage increases for these parameters were 5- to 12-fold greater for TPTD.
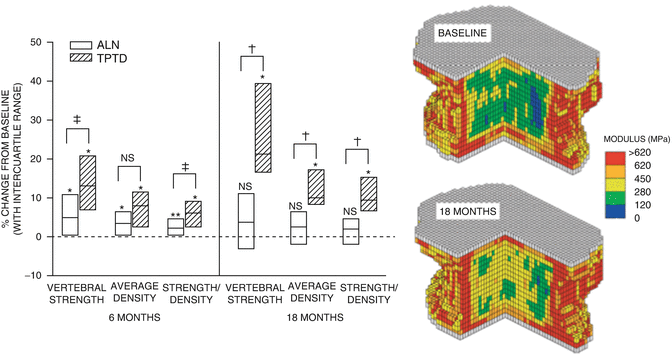

Fig. 3.3
The right panel shows increases in vertebral body compressive strength calculated from finite element analysis (FEA) in patients treated with alendronate (white bars) and teriparatide (hatched bars). Left panel shows the improvements in modulus derived from FEA in a patient at baseline after being treated with TPTD for 18 months. From Keaveny TM et al. Journal of Bone and Mineral Research 2007;22(1):149-57. Reprinted with permission from John Wiley and Sons
Graef et al. [23] studied postmenopausal women with established osteoporosis participating treatment with TPTD for 12 and 24 months in the EUROFORS study. After 12 months, they found an increase in apparent BV/TV (app. BV/TV) by QCT of 30.6 ± 4.4 % (SE), which was paralleled by an increase in apparent trabecular number (app. Tb.N.) by 19.0 ± 3.2 %. These increases were much bigger than those recorded for areal and volumetric BMD—6.4 % and 19.3 %, respectively. After 24 months, they found an increase of app BV/TV by 54.7 ± 8.8 %, while volumetric BMD and areal BMD increased by 19.1 ± 4.0 % and (10.2 ± 1.2 %), respectively. FE analysis of the 24-month data revealed increases in bone strength by 28 % in compression and bending. These results are further corroborated by the virtual absence of severe vertebral (SQ3) fractures in patients treated with PTH (Fig. 3.4).
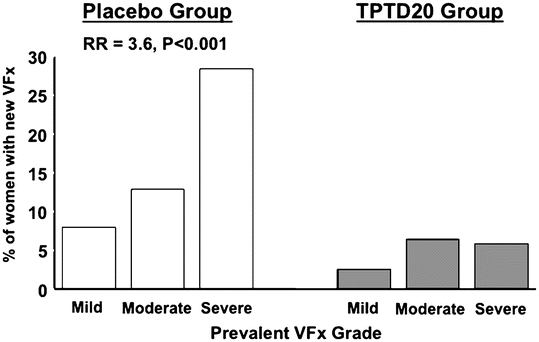

Fig. 3.4
Distribution of vertebral compression fractures classified as either mild (20–25 % compression [SQ1]), moderate (25–40 % compression [SQ2]), and severe (>40 % compression [SQ3]). Based on data from the pivotal PTH(1–34) trial [34]
Effects on Hip Strength
Keaveny et al. [37] performed finite element analysis on the Hip QCT scans of 162 subjects in the PATH trial to assess changes in femoral strength after treatment with alendronate (ALN) or PTH(1–84) or combinations (CMB) thereof in year 1. During year 2, patients were switched to either ALN or placebo (PLC) in the following groups: PTH–PLC, PTH–ALN, CMB–ALN, and ALN–ALN (year 1–year 2) treatments. After year 1, femoral strength increased significantly for both PTH and ALN, and after year 2, significant changes were seen for all groups except PTH–PLC, with the most pronounced increase seen in patients treated with PTH–ALN (7.7 %).
In the EUROFORS study , Borgrefe et al. [24] studied the changes in bone distribution, geometry, and bone strength of the femoral neck (FN) in 52 postmenopausal women with severe osteoporosis. They studied three subgroups: treatment-naïve, pretreated, and pretreated showing an inadequate response to treatment. After 24 months of TPTD treatment, volumetric FN BMD increased significantly by 4.0 % and 3.0 %, respectively, compared with baseline. Decreases in cortical volumetric BMD occurred in locations not adversely affecting minimum bending strength indicators. Cortical cross-sectional area increased by 4.3 % and indicated that endosteal but no periosteal growth was observed. Strength parameters for buckling improved significantly at 24 months. Measures of bending strength showed a trend toward improvement, and the changes tended to be larger in individuals at higher risk of buckling failure. In this study, prior antiresorptive treatment was not found to affect treatment results significantly.
In another analysis of the same EUROFORS cohort, Poole et al. [38] mapped small changes in cortical bone distribution in 69 women with severe osteoporosis after treatment with TPTD for 2 years. Changes in cortical thickness were recorded in each subject by subtracting the baseline thickness distributions from the distributions obtained at 24 months. Interestingly, the analyses demonstrated that new bone was being targeted to regions, which encounter high stress during normal locomotion (i.e., the inferomedial junction of the cortex with the load-bearing calcar femorale and the head–neck junction of the superior cortex)—both sites commonly involved in hip fracture. These findings suggest that mechanical stimulation may enhance local actions of PTH and are in keeping with PTH acting as a modulator of osteocytic mechanosensing and sclerostin secretion as outlined above,
Effects of PTH(1–34) (Teriparatide [TPTD]) in Postmenopausal Osteoporosis
In the pivotal PTH trial by Neer et al., women with severe osteoporosis were treated with subcutaneous injections of placebo or 20 or 40 μg of teriparatide (TPTD) [17]. The average number of fragility fractures per patient was over 2, defining the population as high risk. Over a follow-up period of 21 months, BMD increased by an average of 10–14 %. Femoral neck BMD also improved, but more slowly and to a lesser extent (approximately 3 %). The incidence of new vertebral fractures was reduced by 65 % with the 20 μg dose. The overall incidence of new non-vertebral fractures was reduced by 35 % with the 20 μg dose. When examining non-vertebral low trauma fragility fractures separately, a reduction of 53 % was demonstrable. The higher, 40 μg dose, used in the trial did not further enhance the anti-fracture efficacy. Hip fracture incidence was not analyzed separately because the study was not sufficiently powered to examine this endpoint [34]. When evaluating these results, it is worth noting, however, that the effect sizes emerging were the result of an incomplete trial as the trial was stopped abruptly due to an increased risk of osteosarcoma identified in a long-term toxicology study in rats. This means that the results were obtained in patients with huge variations in treatment duration. The potential anti-fracture efficacy of TPTD in patients treated was shown in a post hoc analysis by Lindsay et al. [1], where patients receiving more than 18 months of treatment with teriparatide revealed a 90 % relative risk reduction in vertebral fractures and a 75–80 % relative risk reduction in non-vertebral fractures (Fig. 3.5). The latter effect should be compared to the 20–25 % reduction seen in the trials using the newer parenteral antiresorptives like zoledronic acid and denosumab [39, 40]. The pronounced effects of TPTD on severe vertebral compression fractures (Fig. 3.6) are probably the main reason behind the significant reduction in back pain demonstrated in several studies [34]. Further post hoc analyses revealed that the reduction in fracture incidence due to teriparatide was not related to the number, severity, or site of previous fractures [41] and was largely independent of age and initial BMD [42].
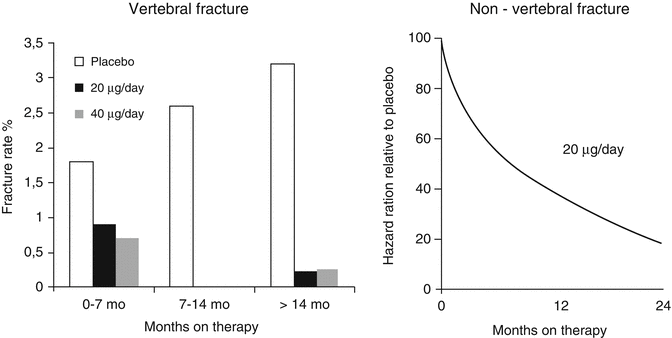
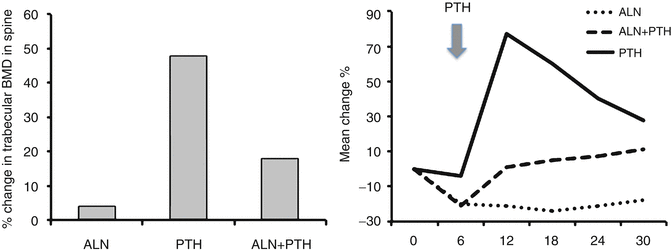

Fig. 3.5
Post hoc analysis of fracture data obtained in the pivotal PTH(1–34) trial by Neer et al. [34]. Vertebral (left panel) and non-vertebral (right panel) fracture incidences in patients treated with the marketed 20 μg dose were analyzed according to duration of treatment in the trial. With increasing duration, a progressive reduction of both fractures was observed. From Lindsay R et al. Osteoporos Int. 2009;20(6):943–8. Reprinted with permission from Springer

Fig. 3.6
Trabecular bone density of lumbar vertebrae as assessed by quantitative computerized tomography (QCT) and bone formation as reflected in serum levels of bone-specific alkaline phosphatase in men treated with alendronate and PTH as monotherapy or combination of the two. Note the reduced BMD and the blunted marker response in the combination group treated with PTH + alendronate. From Finkelstein JS et al. The New England Journal of Medicine. 2003;349(13):1216-26. Reprinted with permission from Massachusetts Medical Society
Two observational studies in Europe, EFOS and EUROFORS, have studied effects of TPTD on back pain and quality of life. With the necessary reservations related to the design of both studies (observational and open label, respectively), significant improvements in bone mass, reductions in back pain, and improvements of quality of life after 18 and up to 36 months of treatment were reported.
The European Forsteo Observational Study (EFOS) enrolled 1648 women with postmenopausal osteoporosis in order to examine the effectiveness of teriparatide. The women were treated for up to 18 months in clinical centers in eight European countries. All participants were TPTD treatment-naïve, but 91.0 % of them had previously received other anti-osteoporosis drugs. After both 18 and 36 months of treatment, significant reduction in back pain and improvements in quality of life were demonstrable [43, 44].
EUROFORS was a prospective, controlled, randomized, open-label, 2-year study, which enrolled 868 postmenopausal women with osteoporosis and a recent fragility fracture. The women were treated at clinical centers around Europe. After 12 months of teriparatide (20 μg/day), 507 patients were randomized to another 12 months with either teriparatide (n = 305) or raloxifene 60 mg/day (n = 100). After 2 years of treatment, TPTD caused a significant increase in BMD of 10.5 % [45] and significant reduction in back pain as assessed with visual analog scales (VAS) [46].
Effects of PTH(1–84) in Postmenopausal Osteoporosis
PTH(1–84) has been the object of a limited number of studies. In a preliminary clinical trial, preparatory to the definitive clinical trial, subjects were administered placebo or 1 of 3 doses of PTH(1–84): 50, 75, or 100 μg for 12 months [47]. These data demonstrated both time- and dose-related increases in lumbar spine BMD. Similar to the teriparatide studies, bone turnover markers rose quickly. Histomorphometric analyses of bone biopsy specimens confirmed an anabolic response to PTH(1–84) with an increase in bone formation and improvements in cancellous bone architecture [48]. PTH(1–84) was found to reduce the risk for new or worsening vertebral fractures by 40 % [49]. Contrary to the results obtained in the teriparatide trial, no reduction of non-vertebral fractures was seen with PTH(1–84), however. This study used a higher overall dose of PTH [100 μg PTH(1–84)], which on a molar basis is equivalent to 40 μg of PTH(1–34). Not surprisingly, this higher dose also resulted in far more adverse events and discontinuations due to hypercalcemia [49]. Moreover, the population tested in this study also had a lower overall risk of osteoporotic fracture. In contrast to the study by Neer et al. in which the average number of fragility fractures in subjects at baseline was >2, the incidence in the PTH(1–84) study was only 19 %. This difference in baseline fracture status, together with the higher dose, may have contributed to the lack of demonstrable effects on non-vertebral fractures.
Teriparatide in Men with Osteoporosis
For most osteoporosis agents , the studies on men are smaller and mainly rely on bridging from studies on changes in bone mineral density in women . In the first study that evaluated the effects of PTH in men, Kurland et al. randomized 23 men to 400 U/day of teriparatide (equivalent to 25 μg/day) or placebo for 18 months [50]. The men treated with teriparatide demonstrated a 13.5 % increase in lumbar spine BMD and a 2.9 % increase in femoral neck BMD. Cortical bone density at the distal radius did not change as compared to placebo. In a larger trial of 437 men by Orwoll et al. [51], BMD increased significantly in the 20 μg treatment group by 5.9 % at the lumbar spine and by 1.5 % at the femoral neck independent of gonadal status. This study was shortened to 11 months due to emergence of osteosarcoma data in rats, but the magnitude and time course of BMD increases at the lumbar spine and hip over the 11 months of the study, were superimposable on the time course seen in the postmenopausal women studied by Neer et al. in the pivotal study [34]. In a follow-up observational period of 30 months, 279 men from the original cohort had lateral spine X-rays 18 months after the treatment was stopped. When combining fracture assessment in the combined treatment groups (20 μg and 40 μg), the risk of vertebral fracture was reduced nonsignificantly by 51 % (P = 0.07), but when only moderate or severe fractures were considered, significant 83 % fracture risk reductions were found (6.8 % vs. 1.1 %; P < 0.02) [52]. When evaluating these fracture reductions, one has to keep in mind that a substantial number (25–30 %) of study subjects reported use of antiresorptive agents during the follow-up period.
PTH in Secondary Osteoporosis
In secondary osteoporosis characterized by severe impairment of bone formation such as in glucocorticoid-induced osteoporosis (GIO) , PTH should theoretically be more effective than antiresorptive agents, because it more specifically affects the primary defect of these diseases being impaired bone formation. This was indeed shown to be the case in a recent study. Saag et al. [53] compared teriparatide with alendronate in 428 women and men with GIO (22–89 years of age) and glucocorticoid treatment for at least 3 months (dose ≥5 mg prednisolone equivalents daily or more). Patients received either teriparatide (20 μg/day) or alendronate (10 mg/day) for 18 months. In the teriparatide group, the increase in BMD was higher than in the alendronate group (7.2 ± 0.7 % vs. 3.4 ± 0.7 %, P < 0.001). Although the trial was not powered to assess differences in fracture rates, pronounced differences in vertebral fractures were demonstrable, however. Patients in the teriparatide group suffered fewer vertebral fractures than patients treated with alendronate (0.6 % vs. 6.1 %, P = 0.004), while the incidence of non-vertebral fractures was similar in the two groups (5.6 % vs. 3.7 %, P = 0.36). In the 18-month extension study, the difference in fracture risk between the two groups was maintained.
The EuroGIOPs trial [54] compared changes in BMD in 92 men (mean age 56 years) who had been treated with glucocorticoids (GC) for ≥3 months and a T-score ≤ −1.5 standard deviations at baseline. Subjects were then randomized to receive either teriparatide or risedronate for 18 months. At endpoint, trabecular BMD by QCT increased by 16.3 % in the TPTD group vs. 3.8 % in subjects treated with risedronate (P = 0.004). High-resolution QCT analyses revealed improvements in both trabecular and cortical structural indices, and finite element analyses showed larger improvements in vertebral strength. Although this study, like the Saag study [55], was insufficiently powered for evaluation of fracture incidence studies, it is noteworthy that none of the patients on teriparatide but five on risedronate developed new clinical fractures (P = 0.056).
Indications for PTH
Teriparatide is used in postmenopausal women and men with osteoporosis who are at high risk for fracture. It is an injectable drug, so patients have to be able to self-administer a daily subcutaneous pen injection. However, the technique of administration of teriparatide has been successfully taught to very old patients. Patients with prevalent osteoporotic fractures before treatment are good candidates for therapy as they carry a much higher risk of fracture as compared to patients without fractures and a T-score below −2.5. Moreover, this risk increases progressively with both the number and severity of fractures. Thus, teriparatide is clearly indicated in severe manifest osteoporosis with multiple prevalent low energy fractures. A very low T-score on its own (e.g., < −3.5), even without an osteoporotic fracture, also confers a high risk for fracture. Patient age is also important as for any T-score, the older the patient, the greater the risk. Due to the high risk of subsequent fracture in patients with multiple (> 3–4) or severe (> 40 % compression) vertebral fractures, PTH should also be considered in these groups. Certainly, patients who fracture while on antiresorptive therapy are good candidates for teriparatide. Other potential candidates for teriparatide include patients for whom one might consider a bisphosphonate, but who cannot tolerate the drug, and finally, severe osteoporosis in younger individuals in the thirties or forties also may constitute an indication for PTH therapy. These individuals have a long life ahead of them, and it seems logical to add bone to the skeleton to reduce their future risk of fracture, instead of just preserving bone mass and stabilizing the skeleton with antiresorptive regimens.
Monitoring PTH Therapy
Assessment of bone mass by DXA in the early phases generally leads to underestimation of new bone formed due to the relative hypomineralization of newly formed bone [21, 56]. The increase in turnover and porosity, which reaches its maximum at 6 months [21], will also contribute to a decreased DXA response early on. Therefore, DXA measurements will tend to underestimate skeletal responses during early phases of PTH therapy. Later DXA measurements will suffer less from the biases associated with the early phases of PTH therapy and are therefore useful after 1 year of therapy as well as during sequential treatment with antiresorptive agents.
Assessment of bone markers is more informative than DXA in the early phases of PTH therapy. PINP (Type I procollagen N-terminal propeptide) has emerged as the most dynamic and specific bone turnover marker for monitoring PTH effects in vivo, but markers like bone-specific alkaline phosphatase and osteocalcin will also increase significantly [57]. Further supporting the use of bone markers is the fact the initial increases between 1 and 6 months in bone markers, in particular PICP and PINP, predict subsequent improvements in bone structure [58] and BMD increases [59, 60].
In the EUROFORS study , Stepan et al. demonstrated significant correlations between 3-month increases in PINP and 24-month increases in Ac.F. Following 3 months of treatment, increases in PINP predicted the increase in Ac.F. (r = 0.52, P < 0.01) and OS (r = 0.54, P < 0.01) after 24 months [61].
Sequential and Combination Therapy with Teriparatide and Antiresorptive Agents
Prior Use of Antiresorptive Drugs
As candidates for anabolic treatment often have been treated with bisphosphonates or other antiresorptive agents, it is important to consider whether such treatment affects the bone-forming effects of PTH.
Prior treatment with raloxifene has been shown to leave the BMD response to TPTD unperturbed, and markers of bone formation increased to a similar extent in patients treated with raloxifene + TPTD and TPTD alone [62, 63].
Cosman et al. treated postmenopausal women , previously given estrogen for at least 1 year, with teriparatide (25 μg) [64]. Lumbar spine BMD increased in a linear fashion during the entire 3-year study and reached a maximum of 13.4 % after 2 years. Moreover, solid stimulation of bone markers was demonstrable. Thus, no blunting of TPTD anabolism was demonstrable.
Pretreatment with alendronate has, however, been shown to blunt PTH anabolism. This is reflected in an inferior BMD response and reduced stimulation of markers of bone formation [63]. Another study, however, showed a good response to teriparatide with rapid increases in BMD [65]. These results imply that the potency of the antiresorptive regimen to control bone turnover can determine the early response to teriparatide. To account for these differences, it is important to note that the baseline bone turnover markers prior to the initiation of teriparatide therapy were markedly different in the two studies. In the study by Ettinger et al., bone turnover markers were almost completely suppressed, while the women in the study by Cosman et al. exhibited less suppression. Thus, it may not be the specific antiresorptive agent used prior to teriparatide , which determines the subsequent skeletal response but rather the extent to which bone turnover is reduced by this agent. To support this idea, the response to teriparatide has been shown to be a function of the level of baseline bone turnover, with higher turnover levels achieving more robust densitometric responses [50].
In the EUROFORS trial , postmenopausal women with established osteoporosis were randomized to receive open-label teriparatide 20 μg/day for the first year. In a post hoc analysis, their BMD response was assessed according to previous osteoporosis treatment as follows: (a) treatment-naïve, (b) prior treatment with an antiresorptive drug with adequate response, and (c) prior antiresorptive treatment with inadequate response (inadequate AR-responders) (n = 421). In all three groups, BMD increased significantly from baseline, but differed slightly between treatment-naïve patients (8.4 %), patients treated adequately with antiresorptive drug (7.1 %), and inadequate responders to antiresorptive therapy (6.2 %). The same trend was seen for total hip BMD, which increased only in treatment-naive patients (1.8 %), while remaining unchanged in the two other groups [66].
Histomorphometric analysis of a subgroup of women in this trial revealed that the stimulation of bone formation was similar in alendronate pretreated and treatment-naive women. Women pretreated with alendronate exhibited increases in activation frequency from 0.11 (cycles/year) at baseline to 0.34 cycles/year after 24 months, while women who were treatment-naïve exhibited increases from 0.19 to 0.33 cycles/year [61].
Thus, the blunting after prior alendronate therapy previously observed in other trials was demonstrable in an open-label study, but less pronounced. This discrepancy may be due to variations in compliance.
Concomitant Use of Anabolic and Antiresorptive Therapy
The initial decreases in BMD seen early after the initiation of PTH therapy at sites rich in cortical have not been associated with increased skeletal fragility at these sites [67]. Nevertheless, some clinicians have considered combination therapy with antiresorptive agents as more beneficial than monotherapy in high-risk patients. Several trials exploring this concept have, however, yielded important data pertaining to this concept [68, 69]. Several groups have published trials using a form of PTH(1–34) or PTH(1–84) alone, alendronate alone, or the combination of PTH and alendronate. Black et al. studied postmenopausal women treated with 100 μg of PTH(1–84)/day [68]. The study by Finkelstein et al. treated men with 40 μg/day of teriparatide [69]. Both studies used both DXA and QCT to measure areal and volumetric BMD. Much to the surprise of proponents of combination therapy, the gains in BMD in patients treated with PTH alone exceeded densitometric gains seen with combination therapy at the lumbar spine and to a lesser degree at the hip (Fig. 3.6). Measurement by QCT, in fact, showed that combination therapy was associated with substantially smaller increases in cancellous bone BMD as compared to monotherapy with PTH. Bone turnover markers exhibited the expected increases and decreases for PTH and alendronate, respectively. Subjects treated with combination therapy, however, revealed bone marker levels in between the two other regimens, indicating a blunting stimulation of bone formation by PTH.
It seems, however, that the mode of administration of the bisphosphonate also plays a role. Cosman et al. compared the BMD and bone marker response to combined therapy with once-yearly zoledronic acid (5 mg) and daily PTH(1–34) with the response seen in patients treated with either agent alone [70]. Contrary to the findings of the studies using orally administered bisphosphonate, the combination of intravenous bisphosphonate and PTH yielded superior BMD responses at the hip and spine over those seen in patients treated with either agent alone (Fig. 3.7). Bone markers revealed an initial reduction of formation markers as seen with oral bisphosphonates, but over time these markers increased and approached markers levels in patients treated with PTH alone within a year, much different from the constant suppression of bone formation seen in the combination group with PTH and alendronate.
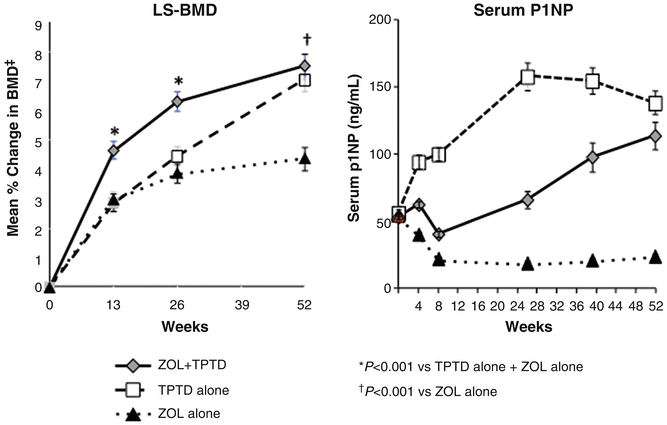

Fig. 3.7
Changes in lumbar spine BMD (LS-BMD) and bone formation as reflected in the bone marker PINP in patients treated with a 15 min infusion of the bisphosphonate zoledronic acid (ZOL) (5 mg) at day 1 followed by 1 year of daily injections with PTH(1–34) (20 μg/day). Contrary to what was seen in the trials testing the combination PTH + alendronate, ZOL did not blunt the BMD response in the combination group (PTH + ZOL). After an initial decrease, bone formation in the combination group picked up and reached the level seen in patients treated with PTH alone. From Cosman F J Bone Miner Res. 2011;26(3):503-11. Reprinted with permission from John Wiley and Sons
Recent data testing the combination of TPTD and denosumab show data similar to those obtained for IV zoledronic acid with the best BMD response in patients treated with the combination of TPTD and denosumab [71]. PINP, however, remained suppressed throughput the treatment period.
Sequential Use of Anabolic and Antiresorptive Therapy
Several different sequential regimens using PTH and bisphosphonates have been published. Cosman et al. studied adding (i.e., concomitant treatment) vs. switching (i.e., sequential treatment) in women either treated with alendronate or raloxifene. They reported that the BMD responses were superior in women where PTH was added, while the biochemical response was better in the switch group. Similarly, Black et al. reported the results of a 2-year study where ibandronate was either given together with PTH for 6 months followed by monthly ibandronate alone for 18 months or PTH was given 3 months every year, followed sequentially by monthly ibandronate. In this study, concomitant therapy led to impaired bone marker responses, while the BMD response was similar in the two groups.
In conclusion, it seems that treatment with weak antiresorptives (raloxifene and estrogen) does not significantly blunt the anabolic effect of PTH. Concomitant and to a lesser degree sequential administration of potent antiresorptive drugs, however, reduces the osteoanabolic action of PTH as reflected in inferior PINP responses. The BMD response is highly variable, with alendronate + PTH showing inferior responses to PTH alone, ibandronate being neutral, while zoledronic acid and denosumab show improvements in BMD response over PTH alone. The improvements in BMD responses seen for the two latter drugs cannot be ascribed to increased anabolism, as PINP is down. Thus, the most likely explanation is a reduction in remodeling space induced by the two drugs. Concomitant treatment with PTH and antiresorptive drugs should therefore be reserved to extreme cases of secondary osteoporosis, where the underlying disease may blunt PTH action (e.g., severe inflammation).
Consequences of Discontinuing PTH Therapy
After discontinuation of PTH, bone mass will return to levels close to baseline within a 2-year period (Fig. 3.8) [67, 72]. Several studies have suggested that this loss of bone mass after discontinuation can be offset by antiresorptive treatment with either bisphosphonate [72, 73], estrogen [74], or raloxifene [45, 62].
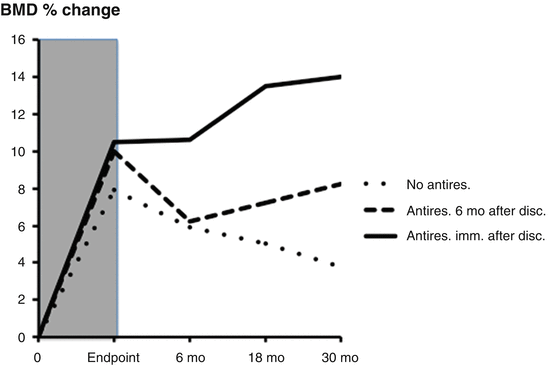

Fig. 3.8
Changes in bone mineral density (BMD) in three groups after discontinuation following therapy with teriparatide (shaded area): (1) no antiresorptive after discontinuation (dotted line), (2) initiation of antiresorptive treatment 6 months after discontinuation (broken line), (3) initiation of antiresorptive immediately after discontinuation (continuous line). Note the additional increase in BMD after antiresorptive treatment. Sixty percent received some kind of osteoporosis treatment after discontinuation, mainly bisphosphonates. From Prince R et al. Journal of Bone and Mineral Research 2005;20(9):1507–13. Reprinted with permission from John Wiley and Sons
The PATH study provided further prospective data to address this issue [75]. In this study, postmenopausal women who had received PTH(1–84) for 12 months were randomly assigned to 12 additional months of therapy with 10 mg of alendronate daily or placebo. In subjects who received alendronate, BMD at the lumbar spine increased further by 4.9 %, while those who received placebo experienced a substantial decline in BMD. By QCT analysis, the net increase over 24 months in cancellous bone BMD among those treated with alendronate after PTH(1–84) was 30 %. In those who received placebo after PTH(1–84), the net change was only 13 %. There were similar differences in hip BMD, with patients treated with alendronate exhibiting 13 % increase vs. 5 % increase in patients on placebo.
Prince et al. studied a cohort of patients for 30 months following the pivotal PTH trial. After discontinuation of PTH(1–34), subjects were given the option of switching to a bisphosphonate or not taking any further medications following teriparatide. A majority (60 %) of patients was treated with antiresorptive therapy after PTH discontinuation [72]. Gains in bone density were maintained in those who chose to begin antiresorptive therapy immediately after teriparatide (Fig. 3.8). Reductions in BMD were progressive throughout the 30-month observational period in subjects who elected not to follow teriparatide with any therapy. In a group who did not begin antiresorptive therapy until 6 months after discontinuation of teriparatide (Fig. 3.8), major reductions in BMD were seen during these first 6 months, but no further reductions were observed after initiation of antiresorptive regimens [67]. Despite the loss of bone mass in a substantial proportion of patients after the discontinuation of teriparatide, vertebral and non-vertebral fracture rates remained reduced for as long as 31 months after discontinuation in women previously treated with PTH (with or without a bisphosphonate) as compared with those treated with placebo (with or without a bisphosphonate; P < 0.03).
Following the initial trial testing of the effect of PTH(1–34) in estrogen-treated postmenopausal women, 52 women were randomly assigned to remain on hormone therapy (HT) alone or continue PTH + HT. Women continuing PTH + HT showed an increase in bone mass over baseline after 3 years by 13.4 % in the spine and by 4.4 % in the total hip. In women discontinuing PTH, but continuing HT, bone density did not increase but remained stable for 1 year after discontinuation without any significant loss, as did bone markers. PTH + HT reduced vertebral fractures from 37.5 % to 8.3 % (P < 0.02).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree