Osteoclast-Targeted Therapy: Bisphosphonates and Denosumab
INTRODUCTION
Bone metastases are a major cause of morbidity in patients with a variety of malignancies including multiple myeloma, breast cancer, and prostate cancer (1). Bone metastases are often described as osteoblastic or osteolytic based on their radiographic appearance. Osteoblastic and osteolytic bone disease represent two extremes of a spectrum, however, and osteoclast number and activity are increased in most bone metastases, including typical osteoblastic metastases from prostate cancer. Pathological activation of osteoclasts appears to play a central role in disease-related skeletal complications.
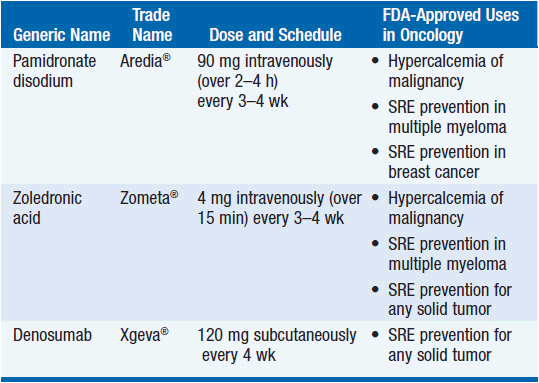
Osteoclast inhibition by treatment with either a bisphosphonate or a denosumab is a standard part of supportive care for many patients with bone metastases (Table 16-1). Bisphosphonates, including pamidronate disodium (Aredia®) and zoledronic acid (Zometa®), are potent inhibitors of osteoclast-mediated bone resorption. Pamidronate disodium and zoledronic acid are approved for the prevention of skeletal-related events (fractures, spinal cord compression, or need for surgery or radiation to bone) in patients with bone metastases from solid tumors or multiple myeloma. Pamidronate disodium and zoledronic acid are also approved for the treatment of hypercalcemia of malignancy. Denosumab (Xgeva®) is a human monoclonal antibody that binds and inactivates receptor activator of nuclear factor-kB ligand (RANKL), a key mediator of osteoclast formation, function, and survival. Denosumab is approved for the prevention of skeletal-related events in patients with bone metastases from solid tumors.
PHARMACOLOGY
 BISPHOSPHONATES
BISPHOSPHONATES
Bisphosphonates are synthetic analogs of pyrophosphate characterized by a phosphorus-carbon-phosphorus backbone that renders them resistant to hydrolysis (Figure 16-1). The R1 and R2 carbon side chains determine their pharmacology. Most bisphosphonates contain a hydroxyl group at the R1 position that confers high-affinity binding to calcium phosphate. The R2 side chain determines antiresorptive potency. Bisphosphonates with a secondary or tertiary amino group at R2 (including zoledronic acid) are among the most potent bisphosphonates, with approximately 10,000-fold greater in vitro potency than etidronate, a first-generation bisphosphonate.
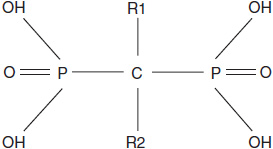
FIGURE 16-1 The molecular structure of bisphosphonates.
Bisphosphonates are adsorbed to calcium phosphate (hydroxyapatite) crystals in bone. Approximately one-half of an intravenously administered dose accumulates in the skeleton. Bisphosphonates preferentially bind to sites of active bone remodeling. Bisphosphonates are not metabolized and are eliminated by renal excretion. Potent nitrogen-containing bisphosphonates (including pamidronate disodium and zoledronic acid) inhibit farnesyl diphosphate synthase, a key enzyme in the mevalonate pathway, and decrease prenylation of essential GTP-binding proteins.
The most common adverse effect of pamidronate disodium and zoledronic acid is an acute phase reaction, a transient flu-like syndrome of fever, arthralgias, and myalgias starting within 24 h after intravenous treatment. Hypocalcemia is also common, but is rarely associated with symptoms. Supplemental calcium (500–1000 mg daily) and vitamin D (at least 400 IU daily) are recommended to reduce the risk of symptomatic hypocalcemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree