Chapter 31 Oropharyngeal Cancer
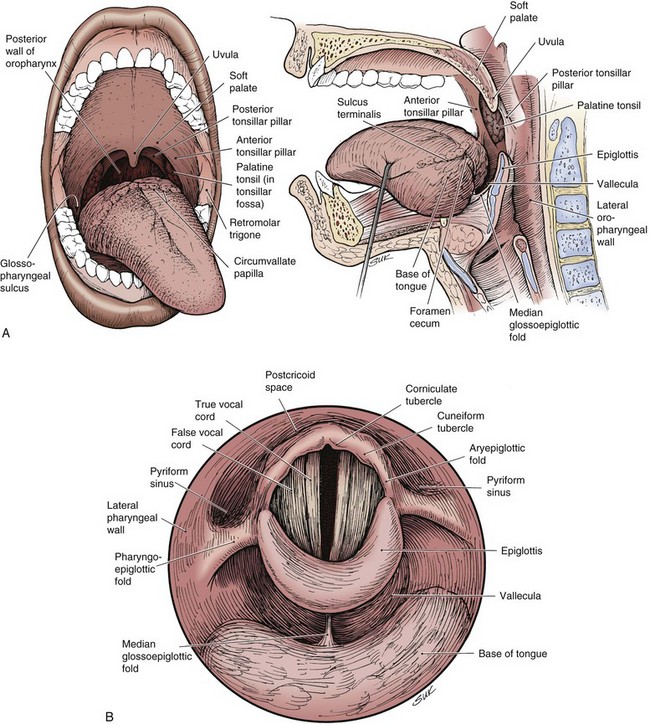
The oropharynx consists of the base of the tongue, tonsillar region, soft palate, and lateral and posterior oropharyngeal walls (Fig. 31-1). Determining the precise annual incidence for new cancers of the oropharynx is difficult because there is overlap with other head and neck sites within various reporting classifications. The cumulative incidence of cancers of the “oral cavity and pharynx,” comprised of the tongue, mouth, pharynx, and oral cavity, was estimated at 36,540 in the United States in 2010, with 25,420 male and 11,120 female patients.1 Malignant tumors within the oropharynx, defined as tumors arising from the base of tongue, tonsillar complex, soft palate, or lateral and posterior pharyngeal walls by the American Joint Committee on Cancer (AJCC) staging definition,2 are generally thought to represent approximately 25% of the total. This places the estimated total between 8000 and 9000 new oropharyngeal carcinomas per year in the United States, although some believe the rate is lower.3
The incidence of head and neck tumors has increased during the twentieth century, particularly in persons born after 1920. The higher cancer incidence over this time period is associated with the rise in alcohol and tobacco consumption in the United States and other westernized countries over the same era.4,5 This trend has reversed over the past two decades in the United States, with a small decline in squamous cell carcinomas of the head and neck, likely secondary to a reduction in tobacco use. Despite this general reduction in head and neck cancers and the diminished incidence rate seen for the neighboring oral cavity, the incidence of oropharyngeal carcinomas has continued to increase since the early 1980s, predominantly in men younger than age 60 years.6,7
The worldwide estimated annual incidence of tumors defined as arising either from the oral cavity or the pharynx excluding the nasopharynx, including oropharyngeal site tumors, was 404,585 in 2002. Over two thirds of the patients were male, and the mortality rate on average was less than half of the incidence rate.8 Such an analysis comes with the typical challenges in documenting such malignant diseases, particularly among the medically underserved in the world. Geographic variations in global rates reflect the prevalence of known risk factors, such as tobacco and alcohol use, within specific populations.
Etiology and Epidemiology
Tobacco and Alcohol Use
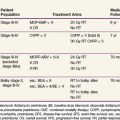
Squamous cell carcinoma of the oropharynx most commonly affects patients older than 50 years, and men more than women. Despite this generalization, over the past 30 years oropharyngeal cancer rates have been on the rise in both men and women under the age of 45 years.7 Both alcohol and tobacco use are long-known, well-defined risk factors for squamous cell carcinomas of the head and neck, including those arising within the oropharynx. Again, separating data regarding oral cavity tumors and oropharyngeal tumors within published series can be challenging given reporting methods, and results may overstate the role of tobacco and alcohol use in oropharyngeal malignant diseases. The two substances individually and together contribute to a field cancerization effect within the upper aerodigestive tract. The effect of alcohol consumption and cigarette smoking on oropharyngeal cancer risk is reported to be dependent on both the intensity and duration of use and is responsible for approximately 80% of oropharyngeal cancers, with a higher proportion in men than in women in one series.9
A population-based study in Sweden compared 605 patients with head and neck cancer with 756 controls. A hazard ratio (HR) of 8.4 was seen with current smokers; this figure was increased by an earlier starting age, longer duration of smoking, greater lifetime total consumption, and increased intensity of daily tobacco use.10 Increasing alcohol intake, particularly more than 50 g per day, was also associated with an increased relative risk of head and neck cancer. A compilation of case-control studies places the tobacco-associated odds ratio at 2.13 for tobacco users when compared with never users, with the ratio doubling in persons who smoke more than 30 cigarettes per day or those who have smoked for more than 30 pack-years.11 Involuntary or secondhand tobacco exposure may also be a risk factor, particularly when the person has been exposed for more than 15 years either at home or at work.12 Alcohol use increases the rate of oropharyngeal carcinomas in never smokers, prior smokers, and current smokers, and the risk decreases with cessation of alcohol use.13 Although use of either alcohol or tobacco elevates the risk of oral cavity and oropharyngeal cancer, there is a synergistic effect from the use of both simultaneously. The risk is calculated to be thirteenfold the expected risk of each used independently, creating an approximately fiftyfold greater risk for oral cavity and oropharyngeal cancer compared with that of nonsmokers and nondrinkers.13,14 Tobacco cessation decreased the risk of head and neck cancer by 40% in years 1 through 4, although the risk of head and neck cancers did not return to baseline until 20 years had elapsed after either tobacco or alcohol cessation.15
Human Papillomavirus Infection
Additional behavioral risk factors have been described besides the classic one of substance abuse, and they appear to be correlated with an elevated risk of oral human papillomavirus (HPV) infection. The role of HPV corresponds to an identified increase in head and neck cancer incidence seen in younger nonsmokers and nondrinkers. The increased incidence has been seen both in the United States and in European countries, despite the general trend of fewer head and neck cancers. This rise has been most marked among young patients in sites associated with HPV.7 HPV is a sexually transmitted disease recently recognized as a risk factor for squamous cell carcinoma of the head and neck, particularly in the oropharyngeal subsite, although the connection was initially suggested in 1983.16 HPV has been implicated in approximately 25% of head and neck cancers based on polymerase chain reaction (PCR) detection of HPV DNA. This prevalence seems to be site specific, with a significantly higher percentage of HPV-associated cancers in the oropharynx compared with the oral cavity or larynx.17 A prospective trial found the rate of HPV-positive tumors to be 60% in the oropharynx overall and even higher in the lingual or palatine tonsils.18 The increase in oropharyngeal cancers is not thought to be entirely attributable to the reduction in tonsillectomy frequency between the 1970s and the 1990s, because this trend would not be expected to alter the incidence of base of tongue primary tumors.19
The link between HPV and oropharyngeal cancers is severalfold and mimics the lines of evidence associating HPV and cervical cancer. First, HPV has been identified in tumor cells of head and neck squamous cell carcinomas.16,20 ![]()
The polymerase chain reaction has been frequently used for identification and subtyping of HPV, as has in situ hybridization via a signal amplification system. Identified prevalence rates have varied, depending on the tumor subsite, sensitivity of the HPV detection method, and geographic location, with associated ethnic variance.2
HPV-16 is the most common HPV subtype associated with oropharyngeal squamous cell carcinomas, seen in 85% to 90% of HPV-associated cancers.17,20 The remainder are associated with HPV subtypes 18, 31, 33, and 35, which play a role in the development of cervical cancer. Second, HPV-positive squamous cell carcinomas of the head and neck have viral characteristics concordant with HPV-associated carcinogenesis, including a high viral load and viral oncoprotein expression.21 The oncogenic function of these HPV-associated oncoproteins ultimately accounts for the different molecular alterations found in HPV-positive tumors compared with HPV-negative tumors.
Additional evidence for an HPV association includes significant links in case-control studies between HPV seropositivity and squamous cell carcinomas of the head and neck. A case-control study comparing 100 patients diagnosed with oropharyngeal cancer and 200 controls found oropharyngeal cancer to be closely associated with oral HPV-16 infection (odds ratio, 14.6), oral infection with any of the HPV types (odds ratio, 12.3), and seropositivity for the HPV-16 L1 capsid protein (odds ratio, 32.2), strongly associating oral HPV with oropharyngeal cancer.22 Behavioral risk factors associated with oral HPV infection have also been correlated with an increased risk of oropharyngeal cancer. They include a high lifetime number of vaginal-sex partners and oral-sex partners.22 This correlation between HPV DNA in tumor specimens and lifetime sex partners has also been identified by others,23 as has age at first intercourse and genital warts.24 ![]()
Patients with HPV-associated head and neck cancers also have an increased risk of anogenital cancers, of which cervical and anal cancers have been strongly associated with HPV infection, and vice versa.25 Again, this supports the influence of sexual behavior and HPV infection on oropharyngeal cancer risk.
Patients with HPV-associated oropharyngeal cancers tend to have distinct clinical and pathologic features compared with those with HPV-negative cancers (Table 31-1). Besides the history of high-risk sexual behavior described above, patients with HPV-associated oropharyngeal cancers are more typically light users or nonusers of alcohol and tobacco,26,27 although this lack of connection has not been universally identified.24 Despite the apparent lack of association between HPV-associated oropharyngeal cancers and tobacco use, there is a correlation with marijuana use and its intensity and duration.27 Individuals with HPV-positive squamous cell carcinoma of the head and neck tend to be younger by approximately 5 years compared with HPV-negative patients.21 Pathologically, HPV-positive cancers are more likely to be poorly differentiated with basaloid features.18
TABLE 31-1 Distinctive Clinicopathologic Characteristics for HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinomas
| HPV-Positive Tumors | HPV-Negative Tumors | |
|---|---|---|
| Anatomic site | Tonsil, base of tongue | All sites |
| Histology | Basaloid | Keratinized |
| Age | Younger | Older |
| Social economic status | High | Low |
| Risk factors | Sexual behavior | Alcohol and tobacco use |
| Survival | Improved | Worse |
| Incidence | Increasing | Decreasing |
| EGFR expression | Negative | Positive |
EGFR, endothelial growth factor receptor; HPV, human papillomavirus.
A meta-analysis of the prognostic impact of HPV in head and neck squamous cell carcinomas demonstrated that HPV-positive patients were likely to have improvements in rates of overall survival (OS) (HR, 0.85) and disease-free survival (DFS) (HR, 0.62). Similar improvements in OS and DFS were identified specifically within the oropharyngeal subsite.28 Improved outcomes with HPV positivity were confirmed in an Eastern Cooperative Oncology Group (ECOG) phase II multicenter trial in which higher response rates were seen after induction chemotherapy and after chemoradiation treatment. This led to statistically improved OS and a lower risk of progression.18 See the Expert Consult website for more information on HPV subtyping, association with cancers, and cancer survival. ![]()
Analysis of the subset of patients with stage III or IV oropharyngeal cancer in Radiation Therapy Oncology Group (RTOG) study 0129 found HPV status to be the major determinant of overall survival (OS), followed by duration of tobacco use and tumor stage.29 Locoregional recurrences were reduced at 3 years (13.6% vs. 35.1%) in HPV-positive tumors, and OS was 82.4% versus 57.1%. Although the bulk of the survival difference was attributable to HPV positivity, favorable prognostic features associated with the HPV-positive subgroup accounted for approximately 10% of the outcome difference.
HPV-positive patients also have a lower risk of second malignant tumors because such patients lack the field cancerization effect produced by alcohol and tobacco exposure.29,30 Given the different clinical, pathologic, and molecular characteristics, as well as the improved prognosis and treatment response, it has been proposed that HPV-positive squamous cell carcinomas of the oropharynx and elsewhere should be considered distinct disease entities from HPV-negative tumors.21,29
Other Risk Factors
Numerous other potential risk factors have been described for oropharyngeal cancer and head and neck cancer in general. A family history of head and neck cancer produces an odds ratio risk increase of 1.7, although this is only seen in subjects exposed to tobacco, suggesting that this factor may represent an environmental as much as a genetic influence.31 Diets low in raw vegetables and citrus and noncitrus fruits have increased the oral cancer risk in a majority of studies.19 These results seem to hold true even when studies have been controlled for alcohol and tobacco usage, although the effect on diet and nutritional deficiencies from heavy alcohol and tobacco abuse is substantial and such factors can be difficult to account for in case-control studies.
Nutritional deficiencies, as signified by a body mass index decline before diagnosis, have also been associated with oral cancer risk. This was again seen primarily among individuals with active or prior alcohol and tobacco use. Poor oral hygiene, as measured by surrogates such as tooth loss and infrequent dental visits, also correlates with an elevated rate of oral and oropharyngeal carcinomas.32 In short, the family history, diet, nutritional status, and oral hygiene may all play a role in oropharyngeal carcinoma predisposition, but defining the impact of each of these in the setting of the more dominant risk factors of alcohol and tobacco use is challenging, particularly given the potential interplay between such factors.
Prevention and Early Detection
Classic prevention for squamous cell carcinoma of the oropharynx, and head and neck cancer in general, has revolved around tobacco cessation and bringing alcohol use to more moderate levels. With the impact of HPV now better understood, ongoing HPV vaccination campaigns to lower the incidence of cervical cancer may simultaneously reduce HPV-related head and neck cancers. Two vaccines are currently available for HPV: one against HPV types 16 and 18, Cervarix (GlaxoSmithKline), and a quadrivalent vaccine against HPV types 16, 18, 6, and 11, Gardasil (Merck). Both vaccines have been advised for women between the ages of 13 and 26 years because they lead to a reduction in precancerous cervical lesions and cancer incidence related to HPV.33,34 Vaccine efficacy in nongynecologic cancers remains to be assessed, but there is optimism that a similar reduction in HPV-associated cancers will be seen in these sites, including the oropharynx.27
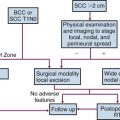
Routine examinations of the oral cavity and proximal oropharynx should be part of a standard medical history and physical and dental examination. Routine oral examinations in high-risk patients have the potential to reduce cancer-related deaths through the discovery of premalignant and early-stage malignant changes. Unfortunately, the same substance abuse conditions that predispose individuals to head and neck cancer correlate with underuse of health and dental services,32 often leading to late detection and diagnosis of head and neck cancers. Radiation oncologists can play a role in the early detection of secondary oral cavity and oropharyngeal cancers attributable to field cancerization in patients who have been treated for primary head and neck cancers and are undergoing follow-up in the radiation oncology clinic.
Potentially malignant lesions include erythroplakia, leukoplakia, erythroleukoplakia, oral submucous fibrosis, and lichen planus. Leukoplakia and erythroplakia are Greek terms translated as “white plaque” and “red plaque,” respectively. Leukoplakia is an exclusionary diagnosis and is currently defined as “a white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer.”35 The worldwide prevalence of leukoplakia is estimated at approximately 2% of the general population,36 with the highest incidence in men from the fifth through the seventh decades; histologic findings show hyperplasia of the squamous epithelium. Most leukoplakia lesions do not progress to squamous cell carcinoma; the annual transformation rate is estimated at 1%.37 This rate is strongly dependent on the presence and severity of dysplasia.38 Dysplasia, carcinoma in situ, or carcinoma is frequently present within erythroplakia, which has only one-sixth of the prevalence of leukoplakia. Of the premalignant lesions, transformation to malignancy is highest in erythroplakia, particularly when dysplastic changes are present, with an annual transformation rate of at least 2.9%.39 Others have predicted that the vast majority of erythroplakias will ultimately undergo malignant transformation.37
Multiple chemopreventive regimens have been evaluated to prevent progression of dysplasia within the upper aerodigestive tract. At least one randomized study demonstrated a decreased rate of biopsy-proven dysplasia after the administration of isotretinoin.40 However, the toxicity was significant, with almost half of patients requiring dose reduction. In addition, most responders relapsed within the first 3 months after treatment. Larger randomized trials found no reduction in the rate of second primary tumors with isotretinoin.41–43
Biologic Characteristics and Molecular Biology
Significant research is ongoing to determine the genetic and molecular impact of HPV on associated oropharyngeal squamous cell carcinomas. Molecular profiles of HPV-positive tumors are different than those of HPV-negative tumors. The oncogenic function of viral E6 and E7 from HPV appears to propel the molecular abnormalities present in HPV-positive tumors. Inactivation of TP53 and PRB is seen with both types, but are the result of separate mechanisms. E6 inactivates TP53 function in HPV-positive tumors, whereas disruptive mutations of TP53 are more common in HPV-negative tumors.44 Besides TP53, disparate effects on 14-3-3σ, RASSF1A, cyclin D, PRB, and P16 have been identified between HPV-positive and HPV-negative tumors. Disruption of TP53 by alcohol- and tobacco-mediated damage seems to be a critical genetic alteration in HPV-negative tumors. A molecular impact of tobacco exposure has also been identified in cells with HPV present. Normal oral keratinocytes transfected with HPV and subsequently exposed to tobacco carcinogens are found to have enhanced E6 and E7, increased resistance to apoptosis, impaired DNA repair, and activated telomerase.44
Endothelial growth factor receptor (EGFR) expression in oropharyngeal cancers correlates inversely with HPV status.45,46 Not surprisingly, given its association with HPV-negative tumors, EGFR expression predicts for a lower rate of response to induction chemotherapy and inferior overall survival and disease-specific survival rates.45
A similar effect was seen in an analysis of the conventional arm of RTOG 90-03, in which 155 patients with adequate pathologic specimens were evaluated for expression of EGFR by immunohistochemical testing.47 EGFR expression did not correlate with T category, N category, or category grouping, but high EGFR-expressing tumors had higher local recurrence rates and worse disease-free and overall survival. Distant metastases did not differ between the two groups.
Pathology and Pathways of Spread
Pathologic Findings
Primary oropharyngeal tumors are almost exclusively squamous cell carcinomas (SCC) (≈95% of tumors). Alternative pathologic types include melanoma (web-only Fig. 31-1)![]() , primary lymphoid malignant tumors (web-only Fig. 31-2)
, primary lymphoid malignant tumors (web-only Fig. 31-2)![]() , minor salivary gland tumors, sarcomas, and other oddities. Given the preponderance of SCC in this site, this chapter will refer to the evaluation and treatment of SCC unless another type is specifically indicated.
, minor salivary gland tumors, sarcomas, and other oddities. Given the preponderance of SCC in this site, this chapter will refer to the evaluation and treatment of SCC unless another type is specifically indicated.
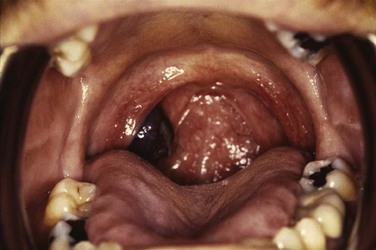
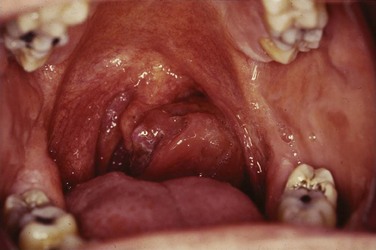
Web-Only Figure 31-1 Hyperpigmented mass arising from the right tonsillar fossa pathologically confirmed as a mucosal melanoma.
A substantial amount of literature has been generated evaluating the histologic subtypes of SCC of the head and neck, the appropriate nomenclature for these tumors, and their clinical impact. Specific subtypes include the spindle cell variant characterized by noncohesive spindle cells, which closely resembles sarcoma microscopically. The basaloid squamous variant is a distinct subtype traditionally correlated with aggressive behavior and poor patient outcomes.48 However, the recent correlation of HPV-associated malignant disease with the basaloid subtype has shown that the basaloid group is a mixture of both HPV-16-positive and HPV-16-negative carcinomas. Because the HPV status imparts a substantially favorable influence on outcome, the implication is that the basaloid phenotype alone does not confer a worse clinical outcome.49 Angiolymphatic invasion and perineural invasion are both associated with increased rates of cervical nodal metastases and a worse prognosis.50
The prototypical SCC of the head and neck, including the oropharynx, is moderately differentiated, with approximately 60% moderately differentiated, 20% well differentiated, and 20% poorly differentiated. Some studies have found tumor grade to be predictive of regional nodal metastasis and, subsequently, clinical outcome. The determination of tumor grade based on traditional cytologic and histologic features of differentiation is challenging to reproduce because of interobserver variability. As a result, the tumor grade seems to have limited prognostic value, particularly when compared with the clinical staging parameters.50 Undifferentiated carcinomas with a lymphoid stroma arising from the tonsil have biologic behavioral characteristics and an increased radiosensitivity that are more similar to undifferentiated nonkeratinizing nasopharyngeal carcinomas rather than conventional types of SCC.51
Pathways of Spread
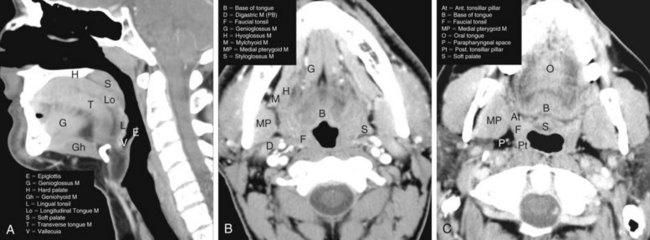
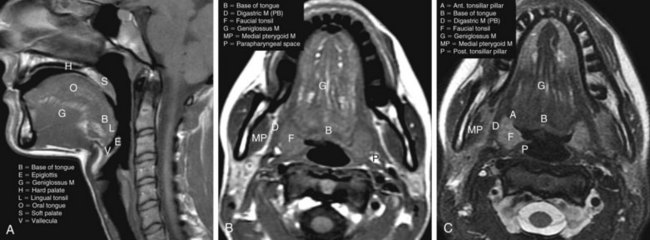
The oropharynx makes up the central portion of the upper aerodigestive tract, encompassing the tongue base, palatine (faucial) tonsils, inferior aspect of the soft palate, and posterior and lateral oropharyngeal walls52 (see Fig. 31-1 and Figs. 31-2 and 31-3). The anterior margins are the anterior tonsillar pillars, the hard-soft palate junction, and the circumvallate papillae of the tongue, placing the posterior third of the tongue within the oropharynx. Superiorly, the oropharynx is bounded by the inferior aspect of the soft palate, posteroinferiorly by the pharyngoepiglottic folds that lie approximately at the level of the hyoid bone, and inferiorly by the glossoepiglottic fold and vallecula. The lateral and posterior borders of the oropharynx are defined by the pharyngeal constrictor muscles and their overlying mucosa. Understanding the anatomy of the oropharynx is necessary when identifying the typical pathways of spread by oropharyngeal neoplasms.53–55
Soft Palate
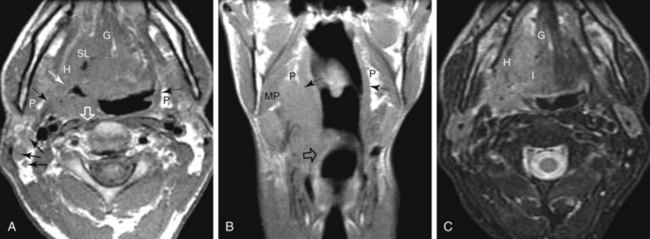
A soft palate tumor can spread anteriorly to involve the hard palate. There is also potential for perineural spread along the greater and lesser palatine branches of the maxillary nerve superiorly into the pterygopalatine fossa56,57 (Fig. 31-4). Further perineural spread from the pterygopalatine fossa can result in extension along branches of the maxillary division of the trigeminal nerve into the orbit via the inferior orbital fissure, into the central skull base via the foramen rotundum, and into the facial nerve and temporal bone via the nerve of the pterygoid canal (vidian nerve). This perineural spread can result in palsies of both the trigeminal and facial nerves. Patients with advanced disease and perineural spread may present with trismus resulting from involvement of the pterygoid muscles or branches of the trigeminal nerve.
Tonsillar Region
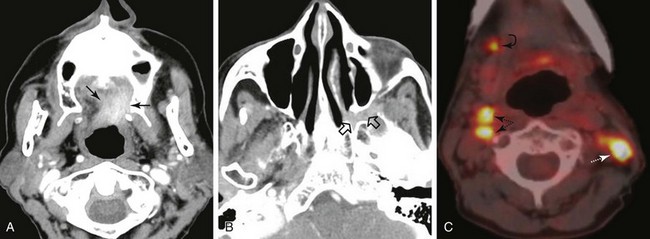
The palatine (faucial) tonsils consist of lymphoid tissue constrained within a fibrous capsule that projects into the oropharynx between the triangle formed by the anterior and posterior tonsillar pillars. The tonsillar fossa is bounded laterally by the superior pharyngeal constrictor muscle. The anterior and posterior tonsillar pillars consist of the mucosa overlying the palatoglossus and palatopharyngeal muscles, respectively. Squamous cell carcinoma is the most frequent neoplasm of the palatine tonsils, followed distantly by non-Hodgkin’s lymphoma. Tumors within the tonsillar region can spread superiorly to the soft palate along the vertically oriented palatoglossus and palatopharyngeal muscles, as well as inferiorly to the tongue base and posterolaterally to the oropharyngeal wall58 (Figs. 31-4 and 31-5).
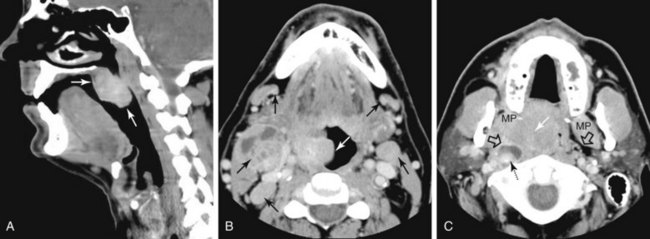
The superior pharyngeal constrictor muscle, in addition to forming the posterior and lateral borders of the oropharynx, courses deep to the palatine tonsils as it extends anteriorly to insert on the pterygomandibular raphe.52 This fibrous raphe is a shared insertion site with the buccinator muscle, with the raphe extending to the medial pterygoid plates superiorly and the lingual cortex of the mandible inferiorly just posterior to the mylohyoid line. Tonsillar neoplasms that invade the superior pharyngeal constrictor muscle and involve the pterygomandibular raphe, therefore, can spread anteriorly to the buccinator muscle and space, superiorly to the medial pterygoid plate and masticator space, and inferiorly to the lingual cortex of the mandible. Lateral extension of tumor allows its entry into the parapharyngeal space (Fig. 31-6). Involvement of the parapharyngeal space by tonsillar neoplasms may result in spread to and along the styloglossus and stylopharyngeal muscles, which extend superiorly toward the skull base and attach to the styloid process. Lateral extension into the medial pterygoid muscle is often associated with involvement of the mandibular division (V3) of the trigeminal nerve. Involvement of V3 can allow perineural tumor spread inferiorly into the mandible along the inferior alveolar nerve and superiorly along the main trunk of V3 to the foramen ovale and cavernous sinus.57
Oropharyngeal Wall
The posterior and lateral walls of the oropharynx are formed by the superior pharyngeal constrictor muscle and overlying mucosa. The deep aspect of the superior constrictor muscle is separated from the prevertebral fascia by a thin layer of retropharyngeal fat. Tumors within the posterior aspect of the oropharynx can extend through this layer of fat and fascia and into the prevertebral space59,60 (see Fig. 31-6). Advanced tumors can ultimately involve the vertebral column, although such spread is typically inhibited by the multiple intervening fascial planes and the longus colli and longus capitis muscles. Tumors of the posterolateral oropharyngeal wall can extend into the nasopharynx and hypopharynx by way of either mucosal or submucosal spread (see Fig. 31-6).
Tongue Base
The tongue base is separated from the oral tongue anteriorly by the circumvallate papillae. It extends posteriorly and inferiorly to terminate near the level of the hyoid bone (see Figs. 31-2 and 31-3). Laterally, it is separated from the palatine tonsils by the glossotonsillar sulcus. The lingual tonsils lie along the posterior surface of the tongue base. The lingual tonsils are variable in size and may be quite large in the first two decades of life and in patients with concurrent head and neck infections.
Neoplasms of the tongue base spread along several pathways as they increase in size. They may infiltrate along the intrinsic muscles of the tongue into the oral tongue and deep tongue musculature. It is especially important to identify involvement of the extrinsic muscles of the tongue (i.e., the hyoglossus, styloglossus, and genioglossus muscles) because this indicates a T4 lesion.61 Tumor may track superolaterally along the mylohyoid muscle to invade the lingual surface of the mandible.62 Deep anterior extension may also involve the hypoglossal or lingual neurovascular bundles. Tumor can track posteriorly along these nerves between the superior and middle pharyngeal constrictor muscles into the parapharyngeal and masticator spaces.
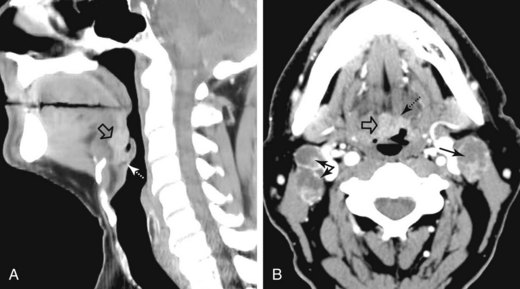
Base of tongue cancer can also spread superficially along the mucosa. Posteroinferior extension can involve the vallecula, lingual surface of the epiglottis, and pharyngoepiglottic folds (Fig. 31-7). There may be invasion of the pre-epiglottic space whenever the vallecula or glossoepiglottic folds are involved.