Introduction
- ■
Mucositis is defined as inflammation of the mucous membranes lining the alimentary tract, causing mucosal injury. Mucositis can occur in response to systemic chemotherapy or other etiologies (e.g., infection, radiation). Mucositis generally begins approximately 3 to 4 days following administration of chemotherapy. , Mucosal injury beyond the oropharynx is discussed in Chapter 6.
- ■
Initial stages of oral mucositis are generally mild, with notable soft tissue erythema and may be associated with burning or mucosal irritation. , Over the following 3 to 5 days, oral mucositis can progress into visible, painful, white desquamative patches advancing to shallow ulcerations due to epithelial sloughing and formation of large painful lesions; most frequently located on buccal tissue, floor of mouth, tongue and soft palate. The severity of oral mucositis ranges from mild to severe, depending on the chemotherapy regimen, and will generally continue for another 3 to 5 days before resolving. Patients receiving concurrent chemoradiotherapy regimens usually develop mucosal soreness by the end of the first week, with ulcerations appearing at the end of the second week. The mucosal ulcerations consolidate to form larger ulcers by the end of the third week and persist for approximately 2 to 4 weeks after completion of radiotherapy, with most ulcers spontaneously resolving without scarring. The more serious forms of oral mucositis are associated with dysphagia and reduced oral intake, and may provoke secondary infections or sepsis, especially in neutropenic patients. ,
Incidence of Oral Mucositis
- ■
Mucositis is one of the most common toxicities of chemotherapy, especially with combination radiation therapy to the mouth or oropharynx. However, the incidence of oral mucositis is generally underreported because toxicity scales are not consistent and oral mucositis is often only reported when considered severe. Table 5.1 describes the incidence of oral mucositis, Table 5.2 describes risk factors for the development of oral mucositis, and Table 5.3 describes the epidemiology of patients with oral mucositis. Notably, targeted therapies, discussed in detail in Part 2, are also complicit in the development of oral mucositis and may compound toxicity when chemotherapy is given in conjunction with radiotherapy.
TABLE 5.1■
Incidence of Oral Mucositis
Clinically Significant Oral Mucositis in Solid Tumor Patients a
Clinically Significant Oral Mucositis in Patients Receiving Chemoradiation and Radiation Therapy b
WHO grade
1: 40%
2: 5%
3, 4: 1%
Mean incidence: 80%
WHO, World Health Organization.
a Prospective study of 298 patients. ,
TABLE 5.2■
Risk Factors Influencing the Frequency or Severity of Oral Mucositis , , , ,
Patient related risk factors
Age (young patients)
Gender (female)
Tumor type (hematological diseases)
Oral health (poor oral hygiene, periodontal disease, dental caries)
Smoking or alcohol use
Nutritional status (malnutrition)
Genetic susceptibility (may play a role)
Kidney and liver function
Chemotherapeutic agents
Drugs that affect DNA synthesis
High doses
Route of administration
Alkylating agents (cyclophosphamide, ifosfamide, bendamustine, melphalan)
Anthracyclines (doxorubicin, daunorubicin)
Antimetabolites (methotrexate, pralatrexate, 5-fluorouracil)
Antitumor antibiotics (bleomycin, mitomycin)
Purine analogues (cytarabine)
Taxanes (paclitaxel, docetaxel)
Topoisomerase inhibitors (irinotecan, topotecan, etoposide)
Secretion into saliva (methotrexate, etoposide)
Other risk factors
Frequency of administration of chemotherapy
Concomitant treatment with radiotherapy
Radiation to head and neck
Bone marrow transplantation
TABLE 5.3■
Epidemiology of Patients Developing Clinically Significant Oral Mucositis
Treatment or Patient Characteristic
Mucositis Risk
Malignancies of the mouth, oropharynx, hypopharynx, larynx, nasopharynx, and salivary glands
70%
Conditioning regimens of high dose melphalan or carmustine, etoposide, cytarabine, melphalan (BEAM) in multiple myeloma or non-Hodgkin’s lymphoma
42%–46% (grades 3–4)
Cyclophosphamide and etoposide
98% (grades 3–4)
Doxorubicin, cyclophosphamide, paclitaxel, or docetaxel in breast cancer
20% (first cycle)
70% (second cycle)
Docetaxel and capecitabine in metastatic breast cancer
60% (grades 1–2)
15% (grades 3–4)
5-Fluorouacil (5-FU) based regimens in colorectal cancer
70% (all grades)
15%–28% (grades 3–4)
Pralatrexate
70% (all grades)
21% (grades 3–4)
Vinflunine for non–small-cell lung cancer
21% (all grades)
Differential Diagnoses
- ■
Fungal (candidiasis) or viral (herpes simplex virus) infection should be considered, especially when heavily keratinized mucosa such as the hard palate, gingiva, or dorsal surface of the tongue is involved.
Etiology and Mechanism of Action of Mucositis
- ■
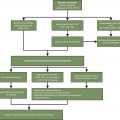
Animal models have greatly improved our understanding of the mechanism behind cytotoxic therapy-related mucositis. This has been described in a five-phase model by Sonis: initiation, primary damage response, signal amplification, ulceration, and healing.
- ■
The initiation phase of mucositis occurs when chemotherapy damages DNA, causing breaks, and generating reactive oxygen species (ROS). This results in the activation of the nuclear factor kappa B (NF-κB) transcription factor. Over 200 genes are managed by NF-κB, including cyclooxygenase-2 (COX-2), and proinflammatory cytokines such as TNF, IL-1β, and IL-6, which have been implicated in the primary damage response phase. , The signal amplification phase occurs as the proinflammatory cytokines accumulate and cause positive feedback loops that continuously activate NF-κB and amplify the response. Ulcerations form as the result of these processes and may be aggravated by oral flora colonization. When there is proliferation of the epithelium around the ulcer, the healing phase has begun, and lesions will spontaneously resolve within 2 weeks of chemotherapy. ,
Grading of Mucositis
- ■
Several grading systems for mucositis have been developed, the most common of which are from the World Health Organization (WHO) and the National Cancer Institute (NCI) ( Table 5.4 ).