CHEMOTHERAPY-INDUCED MUCOSITIS
Approximately 40% of patients undergoing CT develop oral mucositis,11 and approximately half of these patients develop painful lesions requiring parenteral analgesia or total parenteral nutrition (TPN) and treatment modification.12 Incidence rates of 60% are seen in the HSCT setting and 78% for ulcerative oral mucositis.13 Oral mucositis is a risk factor for life-threatening infections for patients with neutropenia. Oral infections, such as herpes simplex virus (HSV) may increase oral mucositis severity. There is a four times greater relative risk of septicemia in patients with oral mucositis and oral infections due to mucosal barrier injury.
The relationship between severe oral mucositis and clinical outcomes in patients who receive conditioning chemotherapy has been reported.14,15 McCann et al.14 conducted an observational study in 197 patients with multiple myeloma (MM) or non-Hodgkin’s lymphoma treated with either high-dose melphalan or BEAM chemotherapy, respectively. Severe oral mucositis (World Health Organization grades 3 and 4) increased TPN duration by 2.7 days, opiates by 4.6 days, and antibiotics by 2.4 days. Oral mucositis presents with asymptomatic erythema and progresses from solitary, white, elevated desquamative patches that are slightly painful to large, contiguous, pseudomembranous, painful lesions. Typical oral sequelae of CT agents include epithelial hyperplasia, collagen and glandular degeneration, epithelial dysplasia, atrophy, and localized or diffuse mucosal ulceration. Nonkeratinized mucosa areas are most affected. The loss of basement membrane epithelial cells exposes underlying connective, innervated tissue stroma, which contributes to more severe oropharyngeal pain.
Oral mucositis is virtually universal when RT targets the oropharyngeal area, with severity dependent on type of ionizing radiation, volume of irradiated tissue, daily and cumulative dose, and duration. Oral mucositis is a dose- and rate-limiting toxicity of RT for head and neck cancer and of hyperfractionated RT and CT. Atrophic changes in the oral epithelium usually occur at total doses of 1,600 to 2,000 cGy, administered at a rate of 200 cGy/day.16 Doses >6,000 cGy or concomitant CT are risk factors for permanent salivary gland changes.16,17 The addition of total-body irradiation to HSCT increases oral mucositis severity through both direct mucosal damage and xerostomia. CT-induced dental effects are related to the treatment field including salivary glands. Teeth in the irradiated field may be desensitized, leading to asymptomatic early caries. Therefore, daily fluoride application is necessary.
Significant health-care costs and resource use are associated with oral mucositis in head and neck cancer.18,19 In a prospective, longitudinal study of 75 patients with head and neck cancer receiving RT with or without CT,19 76% had severe oral pain requiring an increased number of health-care visits, 51% had a feeding tube, and 37% were hospitalized for an average of 4.9 days.
RADIATION THERAPY–RELATED COMPLICATIONS
Long-term effects of head and neck RT include soft tissue fibrosis, trismus, nonhealing or slow-healing mucosal ulcerations, and slow healing dental extraction sites. RT-induced fibrotic changes may occur in the masticatory muscles or the temporal mandibular joint up to 1 year posttherapy. Early phases of fibrogenesis following RT are seen as wound healing characterized by upregulation of tumor necrosis factor (TNF)-α and other proinflammatory cytokines.20 As radiation fibrogenesis continues, it functions as a nonhealing wound.20
Osteoradionecrosis is a relatively uncommon condition related to hypocellularity, hypovascularity, and tissue ischemia. Higher incidences are reported with total doses to the bone exceeding 65 Gy.21 Osteoradionecrosis is usually related to trauma and is reported following tooth extractions not timed to allow extraction site healing for 10 to 14 days before the start of RT. Osteonecrosis of the jaw bone is strongly associated with bisphosphonate use.22,23
PATHOGENESIS OF CHEMOTHERAPY- AND RADIATION-INDUCED ORAL MUCOSITIS
Current knowledge of oral mucositis pathogenesis is informed by translational research that applies advances in molecular-genetic technique and cell biology to this clinical condition.24 Cancer therapy affects rapidly dividing cells including oral basal epithelium with a 7- to 14-day cell turnover rate, thus placing them at risk for CT targeting. Sonis et al.25 reported a “burst” of cyclooxygenase (COX)-2 expression relative to RT-related oral mucositis progression, suggesting that COX-2 plays an amplifying role. In a small pilot study exploring the role of the COX pathway in three patients undergoing autologous HSCT, pain scores significantly correlated with COX-1, microsomal prostaglandin-E synthase, and salivary prostaglandin, suggesting a role for the COX pathway in oral mucositis, possibly via upregulation of proinflammatory prostaglandins.26
Sonis24 has described a five-phase oral mucositis pathogenesis model including initiation, message generation, signaling and amplification, ulceration, and healing. Initiation occurs after administration of CT as a result of DNA damage and generation of reactive oxygen species. The relatively acute inflammatory or vascular phase occurs shortly after CT or RT administration.12 Message generation involves upregulation of transcription factors, including nuclear factor kB and activation of cytokines and stress response genes. Signaling and amplification involves the production of proinflammatory cytokines from epithelial tissue, including TNF-α, which is related to tissue damage, and interleukin (IL)-1, which incites the inflammatory response and increases subepithelial vascularity that may lead to increased local CT levels. Logan et al.27 reported preliminary results in 20 patients undergoing CT demonstrating elevated nuclear factor kB and COX-2 after CT, even though histologically the tissue was similar pre- and post-CT. The epithelial phase shows reduced epithelial renewal and atrophy, and typically begins 4 to 5 days after CT administration. The cell cycle S-phase–specific agents, including methotrexate, 5-FU, and cytarabine, are most efficient for this phase. The ulcerative or bacterial phase begins approximately 1 week post-CT in parallel with maximum neutropenia12; is the most complex, symptomatic phase; and is probably not CT agent class–specific. Patients often experience acute oropharyngeal pain, leading to dysphagia, decreased oral intake, and difficulty speaking. Bacterial colonization of mucosal ulceration occurs, and gram-negative organisms release endotoxins that induce the release of IL-1 and TNF and production of nitric oxide that may increase local mucosal injury. RT and CT are likely to amplify and prolong this proinflammatory cytokine release, leading to exacerbated tissue response. Genetic expression of cytokines and enzymes critical in tissue damage may be modified by transcription factors.12 Fall-Dickson et al.28 described oropharyngeal pain related to oral mucositis in patients undergoing HSCT with CT and measured TNF-α concentration in blood, saliva, and oral buccal brush biopsy samples. Oral mucositis severity was significantly associated with overall intensity of oral pain. TNF-α RNA content in oral buccal brush biopsy samples correlated with worst intensity of oral pain with swallowing. Healing of oral lesions in nonmyelosuppressed patients occurs 2 to 3 weeks after cancer treatment, with decrease in oropharyngeal pain seen in parallel with mucosal healing.
Avivi et al.29 evaluated salivary antioxidant and immunologic capacities in 25 patients with MM treated with conditioning melphalan for HSCT who developed oral mucositis. Oral mucositis was associated with a reduction in secretory immunoglobulin A and antioxidant activity. The increase in salivary Alb and carbonyl indicated mucosal and oxidative damage, respectively.
CHRONIC GRAFT-VERSUS-HOST DISEASE ORAL MANIFESTATIONS
Patients who have undergone allogeneic HSCT (alloHSCT) frequently develop GVHD, an alloimmune condition derived from an immune attack mediated by donor T cells recognizing antigens expressed on normal tissues. GVHD occurs following alloHSCT due to disparities in minor histocompatibility antigens between donor and recipient, inherited independently of HLA genes.30 cGVHD was historically defined as occurring at or beyond 100 days post HSCT,31 and is now classified based on the characteristic clinical presentation.
cGVHD is reported to occur in 30% to 60% of patients more than 100 days post alloHSCT.32 Approximately 80% of patients with extensive cGVHD have some type of oral involvement33 that is a major contributing factor to the morbidity seen with alloHSCT. Although oral lesions are most common with extensive cGVHD, patients may also present with disease involving only the oral cavity, thus making comprehensive oral assessment important. Oral cGVHD presents with tissue atrophy and erythema, lichenoid changes, and pseudomembranous ulcerations, occurring typically on buccal and labial mucosa and the lateral tongue, angular stomatitis, and xerostomia.33 Treister et al.34 correlated the distribution, type, and extent of oral cGVHD lesions with oral pain and discomfort. The buccal and labial mucosa and tongue were the sites of 93% of ulcerations, 72% of erythematous lesions, and 76% of reticular lesions. Ulcerations in the soft palate, although uncommon, were associated with increased pain. Functional impact was significant as seen by restriction of oral intake due to discomfort. Decreased oral intake leads to weight loss and malnutrition, which remain serious problems. Despite the importance of oral cGVHD as a major long-term complication of alloHSCT, little is known about its pathogenesis. Imanguli et al.35 proposed a model of cGVHD pathogenesis in which the production of type 1 interferon by plasmacytoid dendritic cells plays a central role in initiation and continuation of cGVHD. Fall-Dickson et al.36 analyzed associations among clinical characteristics of oral cGVHD and related oral pain and dryness, salivary proinflammatory cytokine IL-6 and IL-1α concentrations, and health-related QOL. Salivary IL-6 correlated with oral cGVHD severity, oral ulceration, and erythema, suggesting its potential utility as a biomarker for active oral cGVHD.
The importance of oral cGVHD was recognized by the National Institutes of Health (NIH) Consensus Development Project on Criteria for Clinical Trials in cGVHD37 and the outcomes of a 2009 conference sponsored by the German-Austrian-Swiss working party for bone marrow and blood stem cell transplantation held in Regensburg, Germany.38 Outcomes of the international conference included a summary of evidence for diagnosis, first- and second-line therapies, topical treatments, and practical treatment guidelines. The authors concurred that a comprehensive, interdisciplinary treatment approach for oral cGVHD must include assessment of mucosa, salivary glands, musculoskeletal tissues, teeth, periodontium, and combined oral function.38 The Schubert Oral Mucositis Rating Scale was validated under the auspices of this NIH Consensus Development Project.37 Treister et al.39 analyzed inter- and intraobserver variability in the component and composite scores using the NIH oral cGVHD Activity Assessment Instrument. Twenty-four clinicians from six major HSCT centers scored high-quality intraoral photographs of 12 patients with a second evaluation 1 week later. Although mean interrater reliability was poor to moderate and unacceptable for the clinical trial setting, greater concordance among the oral medicine experts, high intrarater reliability, and participant feedback suggest formal training may decrease variability. Bassim et al.40 examined the construct validity and internal consistency of the NIH Oral Mucosal Score (OMS) and its individual components (erythema, lichenoid, ulcers, mucoceles), as well as measures of oral pain, function, and related QOL, nutrition, and laboratory parameters in 198 patients diagnosed with cGVHD. The NIH OMS is based only on clinicians’ assessment of oral mucosa in the patient who has undergone peripheral blood stem cell transplantation/bone marrow transplant (BMT). Results supported the construct validity of the discrete components of the NIH OMS as seen through strong associations between erythema and lichenoid and poor oral QOL, function, and nutrition, and lower serum albumin levels. Oral cGVHD ulceration was correlated primarily with oral pain. Validated assessment measures for oral cGVHD are needed to test agents in the clinical trial setting to advance the science of oral cGVHD, and to increase the treatment options.
SEQUELAE OF ORAL COMPLICATIONS
Oropharyngeal Pain
Oral mucositis is the principal etiology of most pain experienced during the 3-week post-BMT time period. This oral pain is often described as the most unforgettable ordeal of BMT. McGuire et al.41 reported in a sample of patients undergoing autologous and allogeneic BMT that pain was reported before observed mucositis, that pain intensity did not correlate directly with extent of mucosal injury, and that some patients reported limited or no pain after BMT. A descriptive, cross-sectional study of women with breast cancer undergoing autologous HSCT conducted by Fall-Dickson et al.42 showed a significant positive correlation between oral pain and oral mucositis severity.
The sensory dimension of oral mucositis–related pain reported with general mucosal inflammation and breakdown ranges from mild discomfort to severe and debilitating pain requiring opioids.43 Oral pain is associated strongly with cGVHD and has been described as severe, burning, and irritating with dryness and loss of taste. Mucositis-related oral pain reported with CT is usually of <3 months’ duration, contrasting with the usually chronic oral pain accompanying oral cGVHD.
Gender differences have been reported for pain. Women reported higher pain levels in a study to test capsaicin efficacy for oral mucositis–related pain. Pain management is critical to avoid suffering and psychological distress.44 Adequate assessment of oral pain requires a comprehensive pain assessment tool such as the Pain-O-Meter (Dola Health Systems, Baltimore, MD),45 which assesses overall intensity, sensory, and affective dimensions of pain.
Xerostomia
Xerostomia experienced by patients who receive head and neck RT is a major sequela, with severity dependent on RT dosage and location and volume of exposed salivary glands. Patients may also develop xerostomia as a late oral complication of HSCT. Brand et al.46 reported in a cross-sectional study of patients with history of autologous or alloHSCT significantly higher levels of xerostomia compared to the comparison group. Xerostomia severity was not significantly associated with RT given before HSCT or the type of HSCT. Xerostomia can affect taste, oral comfort, prosthetic fit, speech, swallowing, and promote caries-producing organisms.
A recent cross-sectional study by Imanguli et al.47 evaluated sicca signs and symptoms in 101 patients with cGVHD using assessment tools used in Sjögren syndrome. Of the 77% of patients reporting xerostomia, those with salivary dysfunction showed histopathologic changes consisting of mononuclear infiltration and fibrosis or atrophy, suggesting that salivary gland involvement is common in cGVHD. A valid, reliable assessment tool is needed to evaluate salivary function in clinical trials. Salivary antioxidant capacity and function were assessed in 30 patients after HSCT by Nagler et al.48 Salivary gland function was assessed measuring total protein, secretory immunoglobulin A, the antioxidant peroxidase, uric acid, and total antioxidant status in a saliva sample. In patients who developed GVHD, there was a significant decrease in salivary flow rate pre- and post-HSCT with no recovery and a reduction in salivary protein content and salivary antioxidant capacity. In patients without GVHD after HSCT, salivary flow rates returned to normal in 3 to 5 months. Decreased salivary flow rate with related decrease in its protective functions for oral mucosa contribute to oral cGVHD severity. Bassim et al.49 tested the feasibility of using liquid chromatography tandem mass spectrometry to identify and elucidate protein biomarkers related to oral cGVHD. Pooled saliva from five patients with moderate or severe oral cGVHD was compared to saliva from a gender- and age-matched pool of five patients with GVHD without oral mucosal findings. Reported reduction in salivary lactoperoxidase, lactotransferrin, and several cysteine proteinase inhibitor family proteins suggests impaired oral antimicrobial host immunity in oral cGVHD.49
STRATEGIES FOR PREVENTION AND TREATMENT OF ORAL COMPLICATIONS
Pretherapy Dental Evaluation and Intervention
Oral or dental stabilization prior to CT and RT is critically important to avoid serious sequela and requires an experienced dental team and informed patients working together for adequate cleaning, elimination oral infection and trauma, and appropriate oral hygiene.50
Patients scheduled for CT or head and neck RT should receive dental screening at least 2 weeks before therapy starts for proper healing of extraction sites, recovery of soft tissue manipulations, and restoration of teeth to promote optimal mucosal health before, during, and following cancer treatment. The initial dental appointment includes examination of dentition for caries and defective restorations, the periodontium, pulp vitality, and denture fit to avoid ill-fitting dentures causing irritation of irradiated tissue and ulceration to underlying bone.51
A panoramic radiograph combined with intraoral radiographs as needed is necessary to detect periodontal disease, periapical infections, cysts, third-molar pathology, unerupted or partially erupted teeth, and residual root tips. Significant oral or dental problems to be addressed before cancer treatment include inappropriate oral hygiene, periapical pathology, third-molar pathology, periodontal disease, defective restorations, dental caries, orthodontic appliances, and ill-fitting prostheses. Bacterial load should be reduced prior to cancer treatment via root planning, scaling, and prophylaxis, excluding visible tumor located at the site of anticipated dental manipulation. The decision to extract asymptomatic teeth prior to the commencement of RT is related to radiation exposure, type, portal field, fractionization, and total dosage in addition to tumor prognosis and expediency of control of the cancer.52 Teeth that are class II or III mobility without use as abutment teeth for prosthetic retention should also be considered for extraction before RT. Extractions of residual root tips and impacted teeth should be performed atraumatically. Alveolectomy and primary wound closure eliminate sharp ridges and bone spicules that could project to the overlying soft tissues. This is important for prosthetic consideration because negligible bone remodeling is predicted after RT. Patients need written instructions for use of oral care agents and instruments for effective daily plaque removal, use of prescribed fluoride treatments, and reportable oral observations and symptoms.
Assessment of the Oral Mucosa
Consistent and frequent oral cavity assessment is needed to assess clinical signs before, during, and after treatment. No standard grading system for severity of oral complications of cancer treatment exists, and available oral complications grading tools are based on two or more clinical parameters combined with functional status, such as eating ability. One commonly used tool is the National Cancer Institute Common Terminology Criteria for Adverse Events v.3.0, which includes descriptive terminology and a severity grading scale for each reportable adverse event.53 Frequently used oral mucosal assessment tools include those that capture both objective and subjective data—the Oral Assessment Guide54 and the World Health Organization Index11,55—and instruments that assess only observed oral changes—the Oral Mucositis Rating Scale,43,56 the Oral Mucositis Index,57 and the Oral Mucositis Assessment Scale (OMAS).58,59 The OMAS was developed as a scoring system for anatomic extent and severity of oral mucositis in clinical research studies.58,59 Oral cavity regions assessed are lip (upper and lower), cheek (right and left), right and lateral tongue, left ventral and lateral tongue, floor of mouth, soft palate or fauces, and hard palate.58 Erythema is rated on a scale from 0 to 2 (0 = none, 1 = not severe, and 2 = severe), and ulceration or pseudomembrane is a combined category rated on scores based on estimated surface area involved (0 = no lesion, 1 = <1 cm2, 2 = 1 cm2 to 3 cm2, and 3 = >3 cm2) and summed with a possible score range of 0 to 162.52,60 Validity and reliability have been demonstrated for the OMAS through clinical research studies.59,61
The optimal treatment strategies for oral complications and related sequelae are unknown. Treatment strategies for oral mucositis and related oropharyngeal pain are mainly empirical, and testing is needed in the randomized controlled clinical trial setting (Table 137.2). Zlotolow and Berger62 presented a comprehensive review of clinical research regarding treatment strategies for oral complications of cancer strategies demonstrating conflicting study results. The only standard forms of care are pretreatment oral or dental stabilization, saline mouthwashes, and oropharyngeal pain management.63
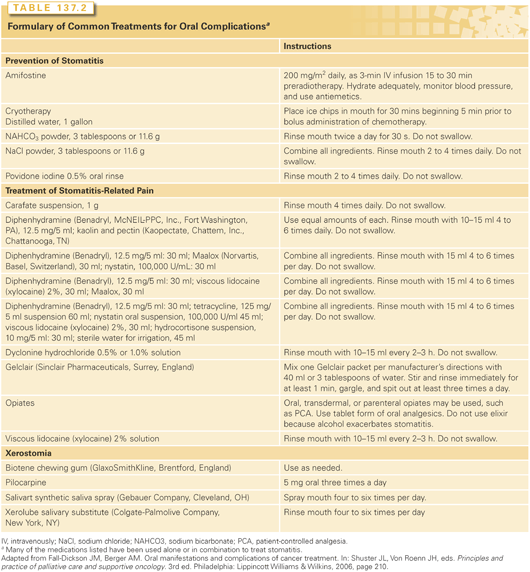
The need for standardized treatment for oral mucositis was appreciated by the Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology through their “Clinical Practice Guidelines for the Prevention and Treatment of Cancer Therapy-Induced Oral and Gastrointestinal Mucositis.”64 These guidelines are based on a comprehensive review of more than 8,000 English-language publications (1966 to 2001) using criteria for level of evidence and quality of research design.65
A standardized approach for the prevention and treatment of CT- and RT-induced oral mucositis is essential. The prophylactic measures usually used for the prevention of oral mucositis include chlorhexidine gluconate, ice-cold water, saline rinses, sodium bicarbonate rinses, acyclovir, and amphotericin B. Regimens used commonly for the treatment of oral mucositis and related pain include a local anesthetic such as lidocaine or dyclonine hydrochloride, magnesium-based antacids, diphenhydramine hydrochloride, nystatin, or sucralfate. These agents are used either alone or in various combinations as a mouthwash formulation. Oral and parenteral opiates are used to relieve oral mucositis-related pain.
Vitamins and Other Antioxidants
Vitamin E has been tested in CT-induced oral mucositis because it can stabilize cellular membranes and may improve herpetic gingivitis, possibly through antioxidant activity. Wadleigh et al.66 demonstrated the efficacy of vitamin E in a sample of 18 patients undergoing CT who were randomized to receive topical vitamin E or placebo.
Other antioxidants that have been tested for efficacy with oral mucositis include vitamin C and glutathione. Osaki et al.67 reported findings from a study of 63 patients with head and neck cancer who were treated with chemoradiation. Twenty-six patients received regimen 1 (vitamins C and E and glutathione) and thirty-seven patients received regimen 2 (regimen 1 plus azelastine). In the azelastine arm, 21 patients remained at grade 1 or 2 oral mucositis, 6 patients had grade 3 oral mucositis, and 10 patients had grade 4 oral mucositis. In the control group, grade 3 or 4 oral mucositis was observed in more than half the patients. Azelastine may be useful to prevent CT-induced oral mucositis.67 Watanabe et al.68 investigated the effect of polaprezinc on CT- and RT-induced oral mucositis, pain, xerostomia, and taste disturbance in patients with head and neck cancer. Thirty-one patients were randomly assigned to polaprezinc or azulene solution as a control for 3 minutes four times a day until the end of the therapy. There was a marked decrease in the incidence of oral mucositis, pain, xerostomia, taste disturbance, and analgesic requirement, and significant increase in food intake in the polaprezinc group.
Zinc sulfate has not been proven to have any benefit in the prevention of RT- and CT-induced oral mucositis when compared to placebo.69,70 In a randomized, double-blind, placebo-controlled trial, Sangthawan et al.69 determined the efficacy of zinc sulfate supplementation in reducing radiation-induced oral mucositis and pharyngitis in patients with head and neck cancer. A total of 144 patients with head and neck cancer receiving RT alone or postoperative RT were enrolled. Zinc sulfate (50 mg) and placebo were administered three times a day at mealtime, on the first day of RT and continued until RT completion. This intervention showed no benefit in relieving radiation-induced oral mucositis and pharyngitis, when compared to placebo.
Amifostine
Amifostine is a thiol compound, selective cytoprotective agent that has been approved by the US Food and Drug Administration for salivary gland protection in patients receiving RT. A retrospective study conducted by Kouloulias et al.71 reported reduced severity of oral and esophageal toxicity. A total of 177 patients with a diversity of tumors were treated with amifostine before RT. Based on a meta-analysis that included patients who received amifostine before RT, there was significant reduction in oral mucositis severity at doses >300 mg/m2.72 A multicenter, open-label, randomized controlled trial analyzed the use of amifostine in patients with MM who received conditioning CT with melphalan prior to autologous HSCT.73 Ninety patients were randomized to receive or not receive amifostine (910 mg/m2). The use of amifostine was associated with a reduction in median grade and frequency of severe oral mucositis. However, there was no reduction in parenteral nutrition and analgesics use and no significant difference between the median progression-free or overall survival times.
Glutamine
Glutamine is an amino acid, immunomodulator, and mucosal protective agent that has been studied in multiple clinical trials with conflicting results. An extensive literature review performed by Savarese et al.74 reported that glutamine supplementation may have an impact on incidence and severity of anthracycline-associated oral mucositis. A randomized, double-blind, placebo-controlled trial on glutamine supplementation in patients who underwent autologous HSCT reported increase in severe oral mucositis and opiate, and prolonged hospital stay.75 Another randomized controlled study comparing oral glutamine supplementation (30 g/day) versus placebo in 58 patients undergoing HSCT reported no difference in length of hospitalization, nutrition, oral mucositis and diarrhea severity, engraftment time, survival, and relapse between both groups.76
Other clinical trials have reported more promising data on the use of glutamine.77,78 A retrospective cohort study including 117 patients treated with RT for cancer of the head and neck or chest tested if oral glutamine prevents oral mucositis or acute radiation-induced esophagitis and favors nutritional status.79 Overall, glutamine was associated with significant reduction of mucositis, weight loss, and enteral nutrition. The risk difference for developing oral mucositis in patients receiving glutamine when compared with controls was −9.0% (95% confidence interval = −18.0% to −1.0%). The majority of patients not receiving glutamine developed severe malnutrition. No differences were seen in interruption of RT, hospitalization, opioid use, or death during RT.
In a double-blind, randomized, placebo-controlled trial of oral glutamine in the prevention of oral mucositis in children undergoing HSCT, 120 patients were randomized to receive glutamine or glycine twice a day until 28 days post-HSCT. The glutamine group showed a significant reduction in days of intravenous opiates use and TPN, but no difference in toxicity was observed between the two groups.80
A phase 3 study of topical AES-14, which is a novel drug system designed to concentrate delivery of l-glutamine to oral mucosa for ulceration treatment, was conducted with 121 patients at risk for oral mucositis.81 Patients were randomized to either AES-14 or placebo and received protocol treatment from day 1 of CT until 2 weeks following the last CT dose or oral mucositis resolution. Results showed a potential 20% reduction of moderate-to-severe oral mucositis in the AES-14 group and a 10% increase in grade 0 oral mucositis.
Anti-Inflammatory Agents
Prostaglandins are a family of naturally occurring eicosanoids, some of which have shown cytoprotective activity. Misoprostol, a synthetic analog of prostaglandin E1, has been studied as prevention and treatment option for oral mucositis due to its anti-inflammatory and mucosa-protecting properties. A randomized, double-blind, placebo-controlled, parallel-group study conducted by Lalla et al.82 evaluated the efficacy of misoprostol oral rinse in reducing the severity of oral mucosal injury caused by high-dose CT. Forty-eight patients receiving myeloablative high-dose CT were randomized to misoprostol (n = 22) or placebo rinse (n = 26). Results showed no significant effect of misoprostol rinse in mucositis.
Topical dinoprostone was administered four times daily in a nonblinded study to 10 patients with oral carcinomas who were receiving 5-FU and mitomycin with concomitant RT.83 The control group comprised 14 patients who were receiving identical treatment. Eight of the ten subjects who received dinoprostone were evaluable; no patient developed severe oral mucositis as compared with six episodes in the control arm. A second pilot study was conducted with 15 patients who received RT to the head and neck, showing that an inflammatory reaction was detected in 5 patients in the vicinity of their tumor when treated with topically applied PGE2, and that no patients developed any bullous or desquamating inflammatory lesions.84 A double-blind, placebo-controlled study of PGE2 in 60 patients undergoing BMT showed no significant differences in incidence, severity, or duration of oral mucositis. Incidence of HSV was higher in the PGE2 arm. Increase in oral mucositis severity was seen in in patients who developed HSV.85
Benzydamine is a nonsteroidal anti-inflammatory drug with reported analgesic, anesthetic, and antimicrobial properties without activity on arachidonic acid metabolism. It has been shown to reduce the severity of oral mucositis and associated pain in patients who undergo RT. Epstein and Stevenson-Moore86 reported in a double-blind, placebo-controlled trial that benzydamine produced statistically significant relief of pain from RT-induced oral mucositis and a reduction in both the total area and the size of ulceration. Positive responses to benzydamine have been reported in three other studies.87–89 In a small prospective, double-blind, randomized study comparing the efficacy of chlorhexidine gluconate and benzydamine hydrochloride oral rinse in patients with head and neck cancer to prevent and treat RT-induced oropharyngeal mucositis, a trend showed decrease in mucositis, oropharyngeal pain, and dysphagia in those receiving benzydamine.90 Given the proposed importance of the prostaglandin pathway in the pathogenesis of oral mucositis, additional studies are warranted.
Current evidence does not support the use of systemic steroids to reduce the frequency or severity of oral mucositis.91
Epidermal Growth Factors
Studies on epidermal growth factor (EGF) as a treatment option for CT- and RT-induced oral mucositis have reported conflicting data. EGF may function as a marker of mucosal damage and could facilitate the healing process.92 In a phase 1 trial conducted by Girdler et al.,93 EGF mouthwash used by patients treated with CT showed onset delay and severity reduction of recurrent ulcerations. No statistical difference was seen in resolution of established ulcers. A double-blind, placebo-controlled, prospective phase 2 study reported potential benefit from EGF oral spray for oral mucositis in patients undergoing RT for head and neck cancer. One hundred and thirteen patients were randomized into one of four arms: EGF-treatment groups (10, 50, and 100 mcg/ml doses twice daily) and placebo. The 50 mcg/ml dose showed the best efficacy.94
Kim et al.95 evaluated efficacy and safety of recombinant human EGF (rhEGF) oral spray for CT-induced oral mucositis with HSCT. Fifty-eight patients were randomly assigned to either the rhEGF group or placebo group. The incidence of National Cancer Institutes grade ≥2 oral mucositis was higher in rhEGF than placebo group (78.6% versus 50%; p = 0.0496), respectively. Duration of mucositis with National Cancer Institutes grade ≥2 was shorter in the rhEGF group (8.5 days versus 14.5 days). rhEGF significantly reduced limitations in swallowing and drinking, and reduced hospitalization duration, administration of TPN, and opioid usage, in patients with grade ≥3. Results were better for patients with advanced oral mucositis in the rhEGF group for several secondary end points.
Hematopoietic Growth Factors
Hematologic growth factors are standard treatment for patients who are treated with high-dose CT because of well-established efficacy to decrease the duration of CT-induced neutropenia. In vitro studies have demonstrated that EGF is present in saliva with ability to affect growth, cell and migration, and repair mechanisms.96 The development of increased oral toxicity or mucosal repair may be dependent on timing of EGF administration in relation to CT treatment.97 Gabrilove et al.98 reported from a sample of 27 patients with bladder cancer who received escalating doses of granulocyte–colony-stimulating factor (G-CSF) during treatment with methotrexate, vinblastine, doxorubicin, and cisplatin. The patients received the G-CSF during the first of two cycles of CT. Although significantly less oral mucositis was seen during cycle one of G-CSF, positive results may be biased related to possible cumulative CT toxicity with resultant increase in oral mucositis severity. Conversely, Bronchud et al.99 reported from a study of 17 patients with breast or ovarian carcinoma treated with escalating doses of doxorubicin with G-CSF that G-CSF did not prevent severe oral mucositis. A third study compared clinical outcomes in 55 adult patients who received CT for non-Hodgkin’s lymphoma and G-CSF with clinical outcomes in 39 patients who received CT alone. Patients who did not receive G-CSF had neutropenia as the primary cause of treatment delay compared with those patients who did receive G-CSF and experienced oral mucositis as the main cause of treatment delay.100 Granulocyte macrophage–colony-stimulating factor (GM-CSF) has demonstrated conflicting results in patients who received diverse cancer treatments.101–104 An open, randomized controlled phase 3 trial conducted by Masucci et al.102 analyzed the efficacy of GM-CSF in patients with head and neck cancer with RT-induced oral mucositis. A significant reduction in oral mucositis severity was observed in the GM-CSF treatment group. Conversely, results from a Radiation Therapy Oncology Group sponsored a double-blind, placebo-controlled, randomized study (n = 121) to analyze efficacy and safety of GM-CSF in reducing severity and duration of oral mucositis and related pain in patients with head and neck cancer receiving RT.104 GM-CSF had no significant effect on severity or duration of oral mucositis.
Keratinocyte Growth Factors
Palifermin, a recombinant human keratinocyte growth factor and member of fibroblast growth factor family, has shown efficacy in reducing oral mucosal injury related to cytotoxic therapy.105 Spielberger et al.105 reported from a double-blind study comparing the effect of palifermin with a placebo for development of oral mucositis in 212 patients with hematologic cancers. Palifermin or placebo was administered intravenously for 3 consecutive days immediately before initiating conditioning therapy with fractionated total-body radiation plus high-dose CT. The palifermin group had significant reductions in grade 4 oral mucositis, soreness of the mouth and throat, opioid use, and incidence of TPN use. Luthi et al.106 reported lower grade 3 or 4 oral mucositis in 34 patients who received melphalan or BEAM with HSCT and were treated with palifermin (0.06 mg/kg) injections 3 days before conditioning CT and 3 days after HSCT compared with controls. Nasilowska-Adamska et al.107 reported palifermin 60 mcg/kg per day for 3 consecutive days before and after conditioning therapy for HSCT significantly reduced incidence, severity, and duration of oral mucositis in 106 patients. In a subsequent study,108 53 patients with hematologic diseases used the same regimen, also showing a significant reduction in incidence and median duration of oral mucositis, decreased incidence of opioid and TPN use, and less prevalence of acute GVHD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








