Chapter 30 Oral Cavity Cancer
The estimated number of newly diagnosed oral cancers in 2010 was 23,880.1 The estimated number of deaths was 5470.
The oral cavity consists of the lips, floor of the mouth, oral tongue (the anterior two-thirds of the tongue), buccal mucosa, upper and lower gingiva, hard palate, and retromolar trigone. Table 30-1 shows the frequency of involvement of various locations.2 After a general discussion of etiology and epidemiology, issues relative to the various subsites will be presented separately. A discussion of preradiotherapy and postradiotherapy dental care is included.
TABLE 30-1 Distribution of Oral Cavity Cancer: 14,253 Cases
| Site | Percentage |
|---|---|
| Lower lip | 38 |
| Tongue | 22 |
| Floor of mouth | 17 |
| Gingiva | 6 |
| Palate | 6 |
| Retromolar trigone | 5 |
| Upper lip | 4 |
| Buccal mucosa | 2 |
Reprinted from Krolls SO, Hoffman S: Squamous cell carcinoma of the oral soft tissues. A statistical analysis of 14,253 cases by age, sex, and race of patients, J Am Dent Assoc 92:571, 1976.
Etiology and Epidemiology
Oral cavity cancer is predominately a disease of middle-aged men who use tobacco and alcohol. Approximately 95% of carcinomas appear after age 45 years, with an average age of 60 years.2 The use of tobacco in any form is associated with an increased risk of oral cancer.3–5 Some evidence suggests that patients with oral cavity cancer who continue to smoke during radiation therapy have poorer outcomes.6 The risk of tobacco-related cancers of the upper aerodigestive tracts declines among former smokers after 5 years, and after 10 years of abstention the risk may approach that of nonsmokers.7 Although the effects of alcohol and tobacco in inducing cancers of the upper aerodigestive tract seem to be additive, the risk of alcohol consumption without tobacco use is unclear. Some studies indicate a slightly increased risk with alcohol use in the absence of tobacco, whereas others show no apparent increased risk.8,9
Human papillomavirus (HPV) infection, marijuana smoking, betel quid use, and drinking the beverage “mate” have also been implicated as causative factors in the formation of squamous cell carcinomas of the upper aerodigestive tract.10–13 In recent years, oral cancers have increased among relatively young females who have never consumed alcohol or smoked. The reason for this is unclear.14
Smokeless tobacco (snuff) can promote carcinomas of the buccal gingival sulcus, which are diagnosed most often in older Caucasian women living in the southeastern United States. Carcinoma of the buccal mucosa is also associated with chewing tobacco. It is commonly seen in the southeastern United States, with a male to female ratio of 3 or 4 to 1.15 Leukoplakia is seen with oral carcinoma in approximately 15% of cases.16
Persons with a “Scotch-Irish” complexion (red hair and blue eyes) and/or prolonged exposure to sunlight are most susceptible to lip carcinoma.17 In one series, 82% were previous or present tobacco smokers.18 Pipe smoking is an alleged risk factor, but this has not been substantiated by most studies. Lip cancer is often associated with poor dental hygiene or edentulous patients.18,19 Lip trauma and a history of alcohol abuse are also related factors.18 Most cases appear after age 40 years, but approximately 10% occur before age 40 years and a few before age 30 years. This disease is uncommon in blacks.
Oral Care
A complete dental examination should be performed on all patients, whether dentate or edentulous, before irradiating any portion of the mandible or maxilla. The radiation oncologist should inform the patient’s dentist of the anticipated radiation treatment plan, including dose and location of the radiotherapy (RT) fields. To make appropriate pretherapy recommendations, the dentist should be familiar with possible postradiotherapy complications, such as caries and osteoradionecrosis. There is a lifelong risk of impaired healing that can lead to osteoradionecrosis, especially when teeth are extracted from hypovascularized and hypocellular bone.20 Therefore, one objective of the pretherapy oral evaluation is to determine whether teeth in the proposed irradiated area can be reasonably maintained in a healthy state for the remainder of the patient’s life.
Medical, dental, and psychosocial issues that affect a person’s future dental health should be assessed at the pretherapy evaluation. The patient’s compliance, motivation for daily oral hygiene procedures, dental awareness, and access to dental care are predictors of dental health. A panoramic radiograph, intraoral radiographs, and hard and soft tissue examinations should be performed to identify high-risk dental factors such as deep caries, nonrestorable teeth, root tips, bony pathology, endodontically treated teeth, periapical and pulpal pathology, and nonfunctional teeth. Teeth exhibiting periodontal disease should be evaluated to determine their long-term prognosis. Some prognostic factors for poor periodontal health include probing depths more than 6 mm, gingival recession, furcation involvement, and mobility.21,22 Because of the numerous reported cases of progression of gingival recession and periodontal disease after RT, it may be difficult to assess the longevity of each tooth.
To reduce the future risk of osteoradionecrosis, teeth with high-risk dental factors should be removed before the patient receives doses of more than 55 Gy.21 Whether extraction of teeth with moderate disease is indicated remains controversial. If the patient has poor resistance to dental disease or an unwillingness to perform routine dental care or fluoride applications, pretherapy extraction of moderately diseased teeth may be justified. A healing time of 14 to 21 days is recommended after extraction, before initiating radiation therapy.20 Extraction should be accomplished as atraumatically as possible, with alveoloplasty to remove sharp, bony projections. The dentist should coordinate dental appointments with the radiation oncologist to minimize the delay in cancer therapy. However, extraction of healthy teeth does not reduce the risk of osteoradionecrosis and should be avoided.23
Clinical practice guidelines for the treatment of cancer therapy-induced oral mucositis have been published.24 Radiation–induced mucositis cannot be prevented; however, excellent oral hygiene can reduce the risk of oral infections. Supersoft toothbrushes and mild toothpastes are available for patients to facilitate proper oral hygiene during and after RT.
Lip Cancers
Anatomy
Lymph vessels from both lips drain into the submandibular lymph nodes. In addition, lymph from the central part of the lower lip drains into the submental lymph nodes. The submental nodes drain either to the submandibular lymph nodes or to the jugulo-omohyoid node. The submandibular lymph nodes drain to the deep cervical chain of lymph nodes.25
Pathology and Patterns of Spread
The most common neoplasms are moderately to well differentiated squamous cell carcinomas; approximately 5% are poorly differentiated.26 Basal cell carcinomas usually arise on the skin above or below the lip and invade the vermilion border, but rarely arise from the vermilion border. Squamous cell carcinomas start on the vermilion of the lower lip, and less commonly on the upper lip. The commissure is rarely the site of origin. Leukoplakia is a common problem on the lower lip and may precede carcinoma by many years.16
Early lesions can initially invade adjacent skin and the orbicularis oris muscle. Advanced lesions can invade the adjacent commissures of the lip and buccal mucosa, the skin and wet mucosa of the lip, the adjacent mandible, and eventually the mental nerve. The incidence of perineural invasion is approximately 2%.27 Lymph node involvement at presentation occurs in approximately 5% to 10% of patients. An additional 5% to 10% of patients with a clinically negative neck subsequently develop lymph node metastases. The risk of lymph node involvement increases with depth of invasion, poor differentiation, larger lesions, invasion of the commissure, and recurrence after prior treatment.19
Hendricks and colleagues28 from the Mayo Clinic reported the following incidence of positive cervical lymph nodes by T stage: T1, 2%; T2, 9%; and T3, 30%. The overall incidence of adenopathy was 19% when the commissure was involved.26 De Visscher and associates29 reported a nodal recurrence rate of 5.4% after primary surgical resection in 184 patients with squamous cell carcinoma of the lower lip. Ninety-three percent of lesions were stage I at presentation.
Clinical Manifestations and Staging
Carcinoma of the lip usually presents as a slowly enlarging exophytic lesion with an elevated border. Occasionally, there is minor bleeding. Erythema of the adjacent skin may suggest dermal lymphatic invasion. Anesthesia or paresthesia of the skin indicates perineural invasion.30
The American Joint Committee on Cancer (AJCC) staging for lip cancer applies to lesions arising from the vermilion surface31 (Table 30-2).
TABLE 30-2 American Joint Committee on Cancer: Oral Cavity Primary Tumor Staging
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor 2 cm or less in greatest dimension |
| T2 | Tumor more than 2 cm but not more than 4 cm in greatest dimension |
| T3 | Tumor more than 4 cm in greatest dimension |
| T4 (lip) | Tumor invades through cortical bone, inferior alveolar nerve, floor of mouth, or skin of face (i.e., chin or nose) |
| T4 (oral cavity) | Tumor invades through cortical bone into deep (extrinsic) muscle of tongue (genioglossus, hyoglossus, palatoglossus, and styloglossus muscles), maxillary sinus, or skin of face |
| T4b | Tumor involves masticator space, pterygoid plate, or skull base and/or encases internal carotid artery |
Reprinted from American Joint Committee on Cancer: Lip and oral cavity. In Edge SB, Byrd D, Compton CC, et al, editors: AJCC Cancer Staging Handbook, ed 7. Chicago, 2010, Springer, pp 49-61.
Treatment
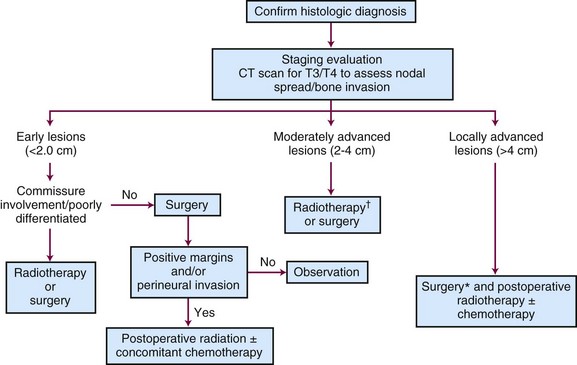
Early Lesions (<2 cm)
The majority of these lesions can be surgically excised with primary closure as an outpatient procedure. Surgery is satisfactory if the lip commissure does not need to be resected and if the resulting aperture of the oral cavity permits the insertion of dentures. Postoperative irradiation is recommended for positive margins or perineural invasion.27,32,33
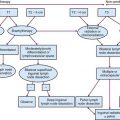
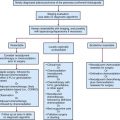
Tumors that should be treated with radiotherapy include those involving a commissure in order to obtain better cosmesis and improved local control.34 The uncommon, poorly differentiated lesions are also preferably treated by irradiation to cover a more generous treatment volume and the first-echelon lymph nodes. An algorithm for treatment planning is shown in Figure 30-1.
Moderately Advanced Lesions (2 to 4 cm)
The length of the lower lip is approximately 7 cm. Removal of more than half of the lower lip with simple closure produces a poor cosmetic and functional result so that a reconstructive procedure is usually necessary. In these cases, irradiation has the advantage of a better functional and cosmetic result. Traditionally, the reconstructed lip may look normal in a photograph but may lack sensory and motor innervation as well as elasticity. However, there have been recent improvements in the functional and cosmetic results of various reconstructive surgical procedures.35–37
Stranc and colleagues34 studied lip function in 37 patients after surgery (19 cases) or radiation therapy (18 cases) and compared results with normal controls. Compared with surgery, irradiation produced better preservation of lip sensation, intercommissural distance, and elasticity. Inadequate lip seal was observed in 2 of 18 patients (11%) after RT compared with 8 of 19 patients (42%) treated surgically.
Teichgraeber and Larson reviewed the M.D. Anderson Hospital experience for patients with lip cancer involving the commissure.38 Of the 22 patients with T2 lesions who were treated with surgery, 10 patients (45%) had recurrence. There are no data for commissure involvement treated with irradiation, but the relative ease of RT and the cosmetic and functional results lead us to recommend irradiation for lesions exhibiting this pattern of spread.
Locally Advanced Lesions (> 4 cm)
Large lesions are managed by resection and postoperative irradiation.33 Erythema of the skin adjacent to the lesion may indicate dermal lymphatic involvement; wide-field irradiation is recommended followed by consideration of surgical resection depending on the response to radiation therapy. Management by definitive radiotherapy and concomitant chemotherapy is generally preferred in patients who are not surgical candidates.
Management of the Neck
Regional lymphatics are not electively treated for T1 and T2 lesions unless commissure involvement is present. Patients with advanced (>4 cm), poorly differentiated, and/or recurrent tumors often require elective neck treatment. Other factors associated with an increased risk of nodal spread include perineural invasion, maximal thickness of more than 6 mm, or low p27Kip1 protein expression.39 The decision to employ elective neck irradiation or elective neck dissection depends on the modality selected for treatment of the primary tumor.
Results
T1 to T3 Lesions
Interstitial Irradiation with or without External Beam Irradiation (EBRT)
Jorgensen and co-workers40 reviewed 869 patients with squamous cell carcinoma who were treated with an interstitial radium implant alone; 90% of the lesions were less than 2 cm in size. The local recurrence and survival rates are shown in Table 30-3; 99% of the primary tumors were ultimately controlled with RT alone or combined with salvage surgery. Thirty-eight percent of patients with T3 tumors who developed a local recurrence developed lymph node metastases. Only 4% of patients died of lip cancer. Twenty-nine complications arose, of which two were bone necrosis.
TABLE 30-3 Lip Carcinoma Treated with Interstitial Radiotherapy (n = 869 Patients)
| Category | 5-yr Local Recurrence | 5-yr Survival |
|---|---|---|
| T1 | 7.4% | 99.5% |
| T2 | 12.7% | 97.4% |
| T3 | 26.4% | 81.4% |
Data from Jorgensen K, Elbrond O, Andersen AP: Carcinoma of the lip. A series of 869 patients, Acta Otolaryngol (Stockh) 75:312-313, 1973.
Pierquin and colleagues41 reported on 50 patients with carcinoma of the lower lip treated with brachytherapy. Only one local recurrence (2%) was observed.
MacKay and Sellers26 reviewed 2854 patients, 92% of whom were initially treated with brachytherapy and EBRT. The primary lesion was controlled in 84% of the cases and 8% were salvaged later for an overall local control rate of 92%. Regional control was achieved in 58% of patients who presented with positive nodes. However, the ultimate rate of neck control was only 35% when neck nodes appeared at a later date. The cause-specific and absolute 5-year survival rates were 89% and 65%, respectively.
Tombolini and associates42 reported on 57 patients with squamous cell carcinoma of the lower lip treated with low-dose-rate brachytherapy alone. Patients with clinically positive neck nodes received EBRT to the involved side of the neck. International Union Against Cancer (UICC) T stages were T1 in 27 cases (47%), T2 in 20 cases (35%), and T3 in 10 cases (18%). The 5-year local control rate was 90%.
Orecchia and colleagues43 treated 47 patients with T1 (n = 21) and T2 (n = 26) lip cancers with iridium-192 (192Ir) brachytherapy. The 5-year and 10-year disease-free survival (DFS) rates were 92% and 85%, respectively.
Guinot and associates44 treated 39 patients with lip carcinoma using high-dose-rate brachytherapy twice daily to total doses ranging from 40.5 to 45 Gy and observed a 3-year local control rate of 88%. Acute and chronic reactions were similar to those observed after low-dose-rate brachytherapy.
Petrovich and associates45 reported on 250 patients with lip cancer treated with RT; half were treated with brachytherapy and the remainder with EBRT. Two hundred forty-seven patients (99%) had squamous cell carcinomas and 240 patients (96%) had lower lip carcinomas. The incidence of lymph node metastasis was 9%. Eleven percent experienced recurrences after irradiation, and half were salvaged; 18 patients (7%) died of lip cancer. Moderately advanced tumors and tumors near the commissures were best treated with EBRT.
EBRT or Surgery
Babington and colleagues33 reported on 130 patients with lip carcinoma; 75% had T1 tumors. Initial treatment consisted of surgery (39%) or RT (48%), or both (13%). Close (≤ 2 mm) or positive margins were observed in 27% of those treated surgically. The 2-year relapse-free survival (RFS) rates were 82% and 54% after RT and surgery, respectively (p < .001). The recurrence rate after surgery was significantly higher for those with close or positive margins.
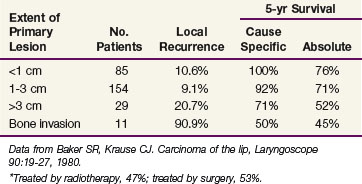
Baker and Krause18 reported on 279 patients treated with either radiotherapy (47%) or surgery (53%) at the University of Michigan (Table 30-4). There was no difference in the 5-year cause-specific survival (CSS) rates between the two groups. Patients with positive regional nodal metastases had a 5-year CSS of 29%. Regional lymph node metastases developed in 31% of those treated for locally recurrent lesions, indicating the need for elective neck treatment for this subset of patients.
De Visscher and associates29 treated 184 patients with squamous cell carcinoma of the lower lip surgically; 93% had stage I cancers. The local and regional recurrence rates were 4.9% and 5.4%, respectively.
Mohs’ Surgery
Mohs and Snow46 reported on 1148 patients with squamous cell carcinomas of the lower lip treated with microscopically controlled surgery. The 5-year local control rates were 94.2% for those with T1 lesions and 59.6% for patients operated on for T2 lesions. The 5-year local control rates were 96.3% for well to moderately differentiated tumors compared with 66.7% for those with poorly differentiated cancers.
Holmkvist and Roenigk47 reported on 50 consecutive patients with squamous cell carcinoma of the lip treated with Mohs’ micrographic surgery (MMS). Four patients (8%) experienced a recurrence; all were successfully salvaged with additional MMS. The average time to recurrence after the initial MMS was 2.5 years. Hruza48 also reported a relatively long interval between MMS and initial recurrence, with 20% of recurrences developing after 5 years.
T4 Lesions
Cancers that present with bone or nerve involvement are usually treated with a combination of surgery and EBRT. There are limited data pertaining to the local control rates after RT or surgery alone, ranging from 0% to 74%26,44; therefore, combined treatment is usually recommended. Byers and colleagues27 observed that 80% of patients with histologically proven perineural invasion developed cervical node metastases. Eight of 25 patients (32%) in their series who presented with either perineural invasion, tumors larger than 3 cm, or regional metastases died of disease.
The postoperative EBRT portals should include the primary site as well as the regional lymphatics (levels IA, IB, and II). The low neck is usually treated to doses sufficient for subclinical disease, and doses are frequently higher in patients with positive nodes. The total dose ranges from 60 to 70 Gy, at 2 Gy per once-daily fraction to the primary site, depending on the pathologic findings. Higher doses with altered fractionation schemes (e.g., 74.4 Gy at 1.2 Gy per fraction twice daily), as well as concomitant chemotherapy, should be considered in patients with positive margins or other high-risk factors such as extracapsular extension.49–51,52,53,54
Recurrent Lesions
Cross and colleagues19 reported on 563 patients treated with surgery for recurrent lip cancer. The prognosis was particularly poor for those with high-grade tumors of whom 16.7% were salvaged compared with 31.8% and 42.9% for those with well and moderately differentiated cancers, respectively. Holmkvist and Roenigk47 reported on four patients treated with MMS; all four were salvaged.
Irradiation Techniques
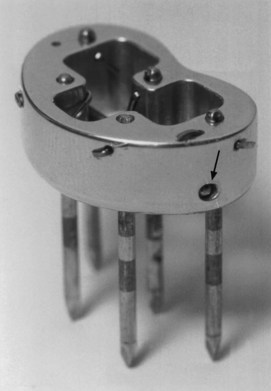
Brachytherapy may be used as the sole treatment or in conjunction with EBRT. Implantation is usually performed under local anesthesia using 192Ir sources and a single-plane plastic tube technique. The sources are arranged horizontally 10 to 12 mm apart with crossing sources on the lateral aspects of the implant. Three to five horizontal sources are used depending on the size of the lesion. The advantage of the plastic tube technique is that the volume of the implant is more easily adapted to the extent of the tumor and the commissure is readily included, if necessary. Alternatively, cesium needles mounted in a nylon bar may be employed (Fig. 30-2). The sources are spaced 1 cm apart and the dose is specified 0.5 cm from the plane of the implant. A gauze roll is placed between the lip and gum to increase the distance between the radioactive sources and the alveolar ridge. The recommended dose is 60 to 70 Gy at a dose rate of 0.4 to 0.5 Gy per hour for an implant alone. Large infiltrative lesions may be first treated with EBRT, 30 Gy at 2.5 Gy per fraction to shrink the tumor, followed by an interstitial brachytherapy boost to deliver an additional 35 to 40 Gy. Treatment of lip cancer with high-dose-rate interstitial needles is advocated by some.44
T3 and T4 Tumors
Low-volume T3 cancers may be treated with primary irradiation, preferably combining EBRT to the primary lesion and neck followed by a brachytherapy boost. EBRT is administered with parallel opposed fields, including the lip lesion and the level I and II lymph nodes55 (Fig. 30-3). A cork is placed in the mouth to displace the maxilla and upper lip and reduce the volume of normal tissue included in the fields. A separate anterior field is used to treat the level III and IV lymph nodes with a tapered midline block over the larynx. The supraclavicular lymph nodes are at low risk and are not included in the fields. Both sides of the neck are treated with irradiation because it is unlikely that T3 and T4 primary lesions would be well lateralized.
Complications
Fitzpatrick56 reported a 3.3% incidence of late complications that required surgical intervention. Orecchia and colleagues43 observed a 10.6% incidence of mucosal necrosis after RT for 47 T1 and T2 lip cancers treated with 192Ir brachytherapy. The risk of late complications increased with dose, dose per fraction, and volume.
Floor of the Mouth
Anatomy
The first-echelon nodes for the floor of mouth are the submandibular lymph nodes (level IB), which may be stratified into preglandular, intraglandular, and postglandular groups. Lymph may also drain into the submental (level IA) nodes. These lymph nodes eventually drain to the jugulo-omohyoid (level II) nodes.25
Pathology and Patterns of Spread
Small to moderate-size (stage T1 and T2) lesions are associated with metastases to the ipsilateral regional lymph nodes in 15% to 38% of cases, depending on the size and depth of invasion of the primary tumor.57,58,59
Mohit-Tabatabai and colleagues60 reviewed 84 patients with squamous cell carcinoma of the floor of the mouth and concluded that lesion thickness was related to the probability of subclinical cervical metastases in clinically node negative (N0) patients (Table 30-5). Patients with a primary lesion more than 1.5 mm thick had a 20% or higher risk of lymph node metastases.60 Histologic grade and configuration of the primary lesion did not have a statistically significant correlation with subsequent development of neck node metastases. O’Brien and associates58 analyzed the impact of tumor thickness on the incidence of regional lymph node metastases and found that the risk increased significantly for lesions of 4 mm or more in thickness. The reported incidence of recurrence in the untreated clinically negative neck varies from 20% to 35%.59,61 Histologic grade and extent of vascular and perineural invasion have been implicated as predictors of lymph node spread.62
TABLE 30-5 Floor of Mouth Carcinomas: Correlation of Primary Tumor Thickness with Neck Failure*
| Thickness (mm) | T1N0 | T2N0 |
|---|---|---|
| 0.1 to 1.5 | 1/38 (3%) | 0/19 |
| 1.6 to 3.5 | 1/5 (20%) | 3/7 (43%) |
| ≥3.6 | 7/11 (64%) | 2/4 (50%) |
* Number of treatment failures/total number of patients.
Reprinted from Mohit-Tabatabai MA, Sobel HJ, Rush BF, Mashberg A: Relation of thickness of floor of mouth stage I and II cancers to regional metastasis, Am J Surg 152:351-353, 1986.
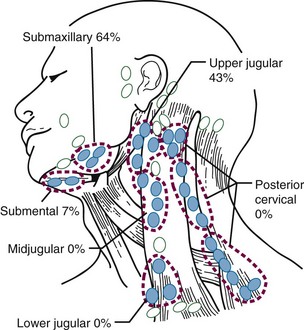
Figure 30-4 shows the distribution of clinically positive neck nodes at diagnosis,63 and Figure 30-5 depicts the distribution of pathologically positive nodes after elective neck dissection in 62 patients with carcinoma of the floor of the mouth.64 The incidence of positive lymph nodes was 19% for those with T1 or T2 lesions and 26% for patients with T3 or T4 cancers.
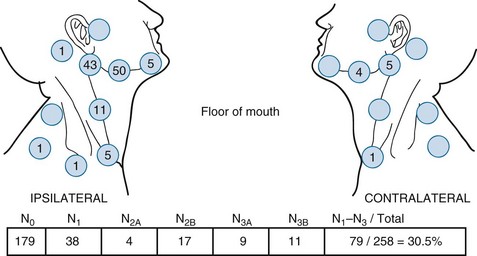
Figure 30-4 Carcinoma of the floor of the mouth. Nodal distribution on admission, M.D. Anderson Hospital, 1948 to 1965.
From Lindberg RD: Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts, Cancer 29:1448, 1972.
Clinical Manifestations, Patient Evaluation, and Staging
The AJCC staging system65 (see Table 30-2) is based on tumor size and invasion of adjacent structures such as bone or soft tissue of the neck. Radiographic studies may facilitate staging with reference to (1) the status of the mandible and teeth, (2) the deep extent of the tumor, and (3) the evaluation of the regional lymph nodes. CT scans should be obtained in essentially all patients. The mandible may be evaluated by panoramic x-ray films, dental films, and CT scanning. Magnetic resonance imaging (MRI) is useful to evaluate marrow space invasion and perineural involvement.66
The role of PET scanning as part of the initial staging workup for oral cavity cancers has not gained widespread acceptance. It may, however, be useful in early detection of recurrences and in prediction of which patients may benefit from elective neck dissection after chemoradiation.67–69
Treatment
Early Lesions (T1 and Superficial T2)
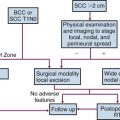
Surgery and radiation therapy produce equal cure rates for T1 and superficial T2 lesions. The risk of irradiation-induced bone and soft tissue necrosis is significant. Therefore, surgery is usually the treatment of choice. The neck is also treated with an elective neck dissection,70 although some advocate observation of the neck in select patients with clinically negative nodes (cN0).71 A treatment algorithm is shown in Figure 30-6.
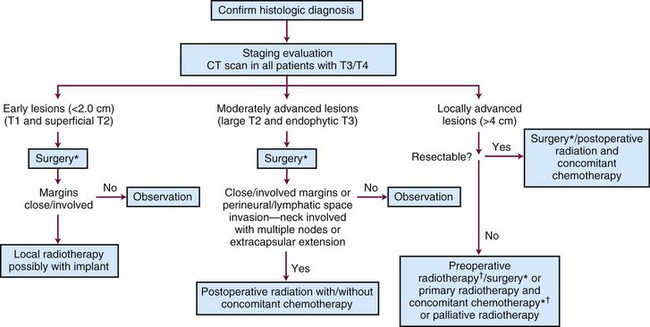
Figure 30-6 Treatment algorithm for de novo floor of mouth cancer. *Treat neck with neck dissection or radiotherapy in any patient with a primary lesion that is more than 1.5 mm thick.60 † Concomitant chemotherapy may be used.154
Sentinel lymph node biopsy is being investigated for possible use in oral cavity cancers.72,73 However, a recent study showed that this procedure was less sensitive for floor of mouth cancers (80%) compared with other oral sites (100%).74
Some patients present after excisional biopsy of the primary tumor. If the margins are either close or involved and there is no evidence of visible or palpable residual tumor, an interstitial implant alone to the primary site is a good alternative provided that the depth of invasion is less than 1.5 mm. Re-excision may not be feasible, because the surgeon does not know exactly what to remove. An additional advantage of RT is the ability to treat a larger area. Six patients were treated in this manner at M.D. Anderson Hospital75 and seven patients at the University of Florida.76 All had disease locally controlled, and none of the patients developed regional lymph node metastases. Conversely, Sessions and colleagues71 have recommended re-excision rather than irradiation if close or positive margins are found on permanent sections.
Moderately Advanced Lesions (Large T2 and Exophytic T3)
One common indication for adjuvant RT is the inability of the surgeon to obtain adequate margins of resection, because this often leads to local recurrence,77 even if immediate re-excision is performed.78 Jacobs and associates79 and Laramore and colleagues80 reported a large intergroup study where adjuvant postoperative EBRT (60 Gy) was administered for locally advanced cancers. They found that the relapse rate was 11% in patients with satisfactory margins and 26% in those with unsatisfactory margins. Unsatisfactory margins tend to reflect a higher residual tumor burden; therefore it may be prudent to deliver a higher dose of postoperative RT. At the University of Florida, patients with involved margins receive hyperfractionated irradiation to increase the dose given to the primary site while minimizing the potential late morbidity.52
Lapeyre and colleagues81 reported on 36 patients with oral tongue or floor of mouth carcinomas treated with brachytherapy after resection with close or positive margins. The local control rate was 88.5% at 2 years. Grade 2 to 3 chronic sequelae were seen in 3 of 19 patients (16%) with floor of mouth cancers.
The neck should be electively dissected if it is clinically negative. If there are multiple positive nodes or extracapsular extension, postoperative irradiation is indicated.32,52 Based on the latest results of the European Organization for Research and Treatment of Cancer (EORTC) and Radiation Therapy Oncology Group (RTOG) trials, concomitant chemotherapy is generally recommended as well in high-risk postoperative settings such as tumors with positive margins and/or extracapsular extension.49,51
Concomitant Postoperative Chemoradiation
The issue of whether concomitant chemotherapy is beneficial when administered with postoperative irradiation for head and neck cancer was recently addressed by two randomized trials (RTOG 9501 and EORTC 22931).49,51 Each of these showed an improvement in locoregional control and DFS when cisplatin (100 mg/m2) was given on days 1, 22, and 43 of the EBRT regimen.49,51 Severe acute effects are seen more frequently with chemoradiation compared with postoperative RT alone.
Results
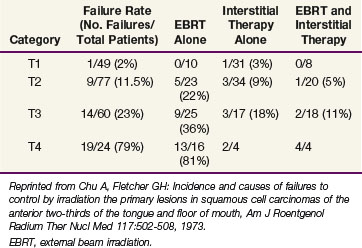
Outcomes after radiation therapy alone vary with the stage of disease and treatment technique. The irradiation schedules used at the University of Florida are shown in Table 30-6.82 EBRT alone results in lower local control rates compared with brachytherapy alone or combined with EBRT.83 Table 30-7 provides the M.D. Anderson Hospital experience with RT alone.84 The failure rates range from 2% for T1 lesions to 23% and 79% for T3 and T4 lesions, respectively. For T2 and T3 lesions, brachytherapy with or without EBRT appears to result in better local control rates than EBRT alone.
TABLE 30-6 Carcinoma of the Floor of Mouth: Radiation Therapy Schedules Currently Prescribed at the University of Florida
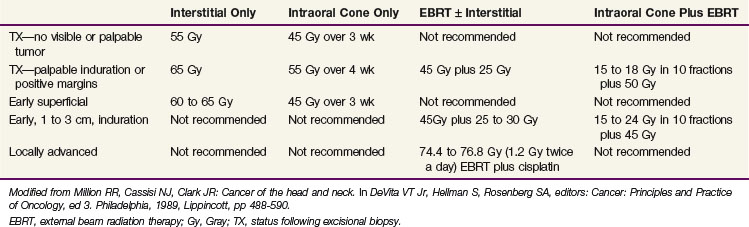
TABLE 30-7 Floor of Mouth Cancer: Failure to Control the Primary Lesion versus Radiation Therapy Technique (M.D. Anderson Hospital, January 1948 through December 1968)
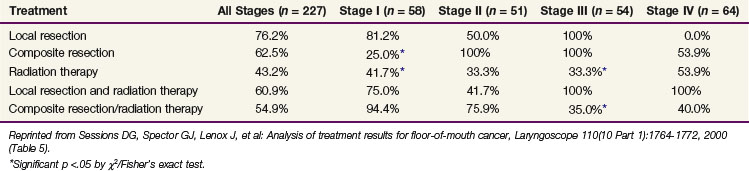
Sessions and colleagues71 reported on 280 patients who were treated with surgery alone, RT alone, or combined surgery and RT. Tumor extent was T1, 106 patients; T2, 107 patients; T3, 40 patients; and T4, 27 patients. The local recurrence rate was 41%. The 5-year CSS by treatment modality are shown in Table 30-8.
TABLE 30-8 Floor of Mouth Carcinoma: 5-yr Cause-Specific Survival Rates versus Treatment Modality and cTNM Stages (Washington University) (n = 227 Patients)
One hundred sixty patients with T1 (79 patients) and T2 (81 patients) floor of mouth cancers were treated at the Institut Gustave-Roussy with low-dose-rate 192Ir brachytherapy.85 One hundred twenty-seven patients had a clinically negative neck and 33 patients (21%) had N1 neck disease. Patients with T2 and/or N1 lesions underwent a neck dissection. With a minimum follow-up of 9 years, local control rates were 93% for T1 cancers and 88% for T2 tumors.
Pernot and associates86 reported on 207 patients with floor of mouth carcinomas treated with EBRT and 192Ir brachytherapy (105 patients) or brachytherapy alone (102 patients). Tumor stages were 41%, Tl; 48%, T2; 8%, T3; 2%, T4; and 1%, TX. Neck stages were 83%, N0; 12%, Nl; 3%, N2; and 2%, N3. The 5-year local control rates were 97%, 72%, and 51% for Tl, T2, and T3 tumors, respectively. The 5-year CSS were T1, 88%; T2, 47%; and T3, 36%.
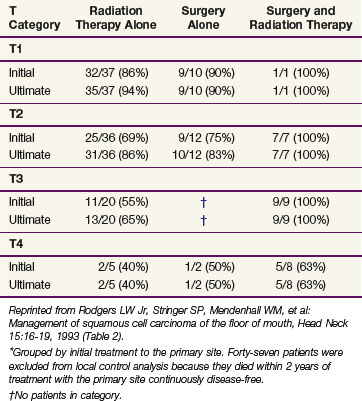
Rodgers and associates87 reviewed 194 patients treated at the University of Florida. Patients with advanced lesions (T3 or T4) had lower control rates when treated with RT or surgery alone compared with those treated with combined surgery and RT (Table 30-9). The 5-year CSS were stage I, 96%; stage II, 70%; stage III, 67%; and stage IVA, 44%.
TABLE 30-9 Floor of Mouth Carcinoma: Initial and Ultimate Local Control Rates*
(University of Florida) (n = 194 Patients)
Irradiation Techniques
T1 and T2 Cancers
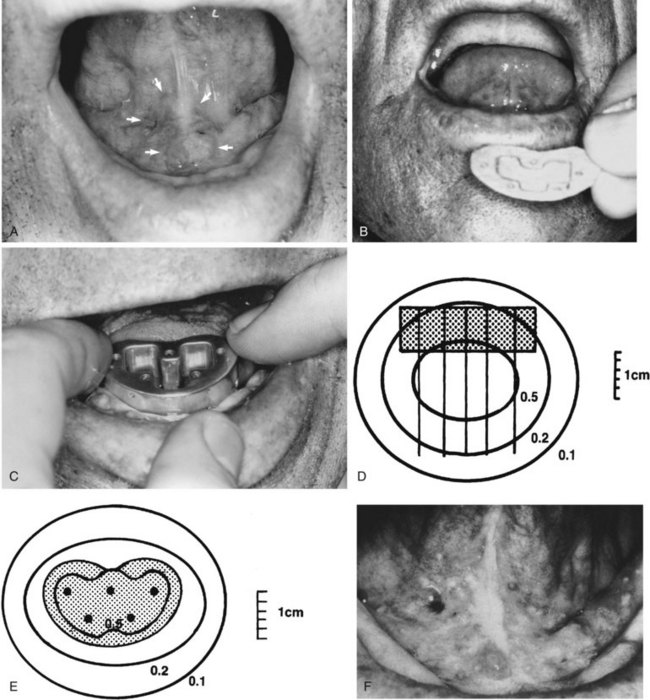
Patients with superficial (≤4 mm thick), well-differentiated squamous cell carcinomas of the floor of the mouth may be treated either with brachytherapy alone or, when accessible, with intraoral cone irradiation. Brachytherapy is not feasible if the tumor abuts or extends onto the mandibular alveolar ridge because of the risk of bone exposure. Brachytherapy may be performed with either rigid cesium needles mounted in a customized template or with iridium using the plastic tube technique. The rigid needles are preferable because although the needles are active, the implant can be accomplished rapidly because the needles are mounted in a rigid template88,89 (Figs. 30-7 and 30-8). An additional advantage of this technique is that the geometry of the implant is optimal and dosimetry can be obtained before the implant. The vertical needles are spaced approximately 1 cm apart with a crosser to ensure an adequate surface dose. The implant is anchored in place by a suture placed through the submentum into the floor of the mouth.
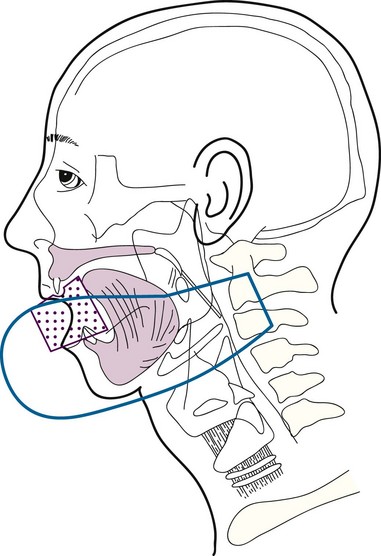
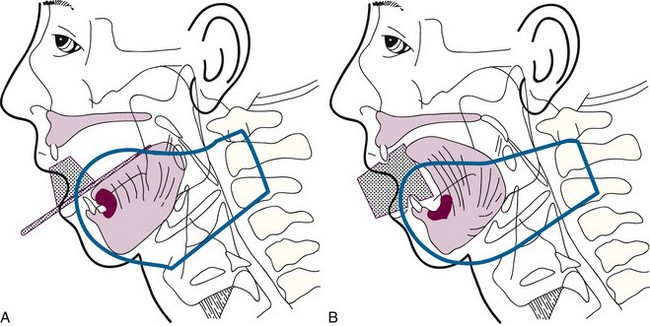
Cancers thicker than 4 mm and those that are poorly differentiated have an increased risk of subclinical disease in the regional nodes. The first-echelon nodes for the floor of the mouth are the level I and II nodes. EBRT is delivered with either 4-MV or 6-MV x-rays using parallel opposed fields that encompass the primary tumor as well as the first-echelon nodes. An intraoral stent is placed in the mouth to displace the maxilla and upper lip out of the fields55 (Fig. 30-9). The EBRT fields are treated to 46 Gy in 23 fractions once daily or 38.4 Gy at 1.6 Gy per fraction twice daily. Brachytherapy follows the EBRT if that is the technique selected to boost the tumor. If intraoral cone radiation therapy is selected to boost the tumor, it precedes the EBRT so the extent of the tumor can be optimally defined and because it may be difficult to place the cone after EBRT because of patient discomfort. The total dose ranges from 65 to 70 Gy.