Fig. 1
Cumulative incidence of recurrent VTE after the first episode of DVT and/or PE. The incidence of recurrent VTE after discontinuing anticoagulation is twice as high in patients with idiopathic than in those with secondary VTE
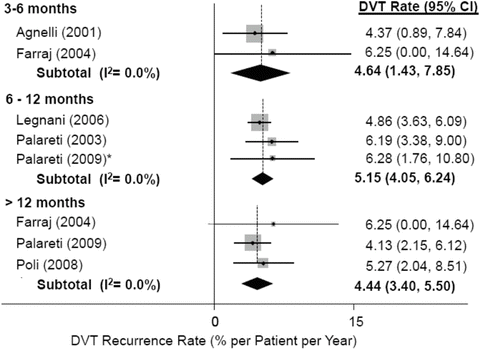
Fig. 2
DVT recurrence rates after treatment cessation. Whichever the duration of anticoagulation (up to 6 months, up to 12 months, longer than 12 months), the incidence of recurrent DVT remains unchanged
1.1 Provoked Versus Unprovoked VTE
The risk of VTE recurrence is negligibly low after major surgery or trauma (annual rate, lower than 1.0 %), whereas it remains substantial if the thrombotic episode is triggered by “minor” transient factors such as hormonal treatment, minor injury, pregnancy, puerperium or long trips (annual rate, around 4.0 %) (Baglin et al. 2003). Patients with permanent risk factors (active cancer, longstanding immobilization, antiphospholipid syndrome) and those without apparent explanations for their thrombotic episode have a remarkable higher risk (annual rate, higher than 7.0 %) (Prandoni et al. 1996, 2007; Schulman et al. 1995; Hansson et al. 2000; Baglin et al. 2003).
1.2 Clinical Characteristics
Single studies and meta-analyses have consistently shown that the risk of recurrent VTE is approximately 1.5 times as high in men than in women (Kyrle et al. 2004; McRae et al. 2006; Douketis et al. 2011). The discrepancy with the gender-specific risk of the first VTE episode is apparent, and most probably explained by sex-specific risk factors at time of first venous thrombosis that are removed later on (Lijfering et al. 2009; Le Gal et al. 2010). Indeed, when female reproductive risk factors are taken into account, the risk of a first venous thrombosis is twice as high in men as in women as well (Roach et al. 2014).
Old age, which is a well-known risk factor of venous thrombosis, has recently been identified – although not consistently (Eischer et al. 2009) – as a predictive factor of recurrent VTE (Prandoni et al. 2007). Accordingly, the common practice of giving old patients lower doses or shorter periods of anticoagulation should be reconsidered (López-Jiménez et al. 2006).
Overweight was recently found to be a powerful and independent risk factor of recurrent VTE (Eichinger et al. 2008). Obese patients should, therefore, be carefully educated, as weight-loss is likely to play a key role in reducing the risk of relapses.
Finally, in a recent cohort study non-0 blood type has been found to be an independent predictor of recurrent VTE (Gándara et al. 2013). This information, which can be easily obtained, has the potential to be incorporated in stratification models addressing the risk of recurrent VTE.
1.3 Modality of VTE Presentation
Patients with a first symptomatic unprovoked deep vein thrombosis (DVT) are at higher risk of recurrent VTE than those with a first unprovoked pulmonary embolism (PE) (Kovacs et al. 2010). In addition, patients with symptomatic PE have a risk of recurrent PE that is 2–3-fold higher than those with DVT alone. These findings, which have recently been confirmed by a patient-level meta-analysis (Baglin et al. 2010), should be taken into account when deciding the optimal duration of anticoagulation. Indeed, a PE episode is potentially more dangerous than an event confined to the leg veins.
2 Inherited Thrombophilia and Family History
Inherited thrombophilia does not increase the risk of recurrent thromboembolism while on anticoagulation (Kearon et al. 2008), while its role in recurrent VTE once anticoagulation is stopped is controversial. It is generally accepted, although not conclusively demonstrated, that carriers of (even mild) antithrombin, protein C and S defects (De Stefano et al. 2006; Di Minno et al. 2014), carriers of hyperhomocysteinemia (Eichinger et al. 1998) and carriers of increased levels of factor VIII or IX (Kyrle et al. 2000; Eischer et al. 2008; Weltermann et al. 2003) have a recurrence risk that is higher than that of control subjects. It is, instead, matter of debate if carriers of factor V Leiden or prothrombin G20210A variant have a higher risk of recurrence as well, as there are data in favour and against this association (Segal et al. 2009; Lijfering et al. 2010). As a consequence, whether detection of these abnormalities, which are highly prevalent in western countries, has the potential to identify subgroups of patients who might benefit from individually adjusted prevention strategies, is uncertain.
3 Post-Baseline Factors
3.1 D-Dimer
Several studies consistently suggested that positivity of D-dimer at the time of warfarin discontinuation or soon after its interruption helps identify patients at a higher risk of developing recurrent VTE (Palareti et al. 2002, 2003; Eichinger et al. 2003; Verhovsek et al. 2008). These findings have recently been confirmed by a meta-analysis of available investigations (Bruinstroop et al. 2009) (Fig. 3).
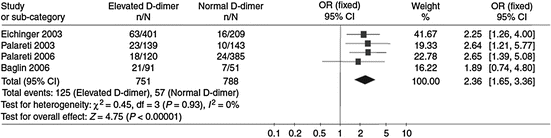

Fig. 3
D-dimer and the incidence of recurrent VTE. The positivity of D-dimer after discontinuing anticoagulation consistently predicts the development of recurrent VTE
3.2 Post-Thrombotic Syndrome
Another post-baseline factor potentially associated with an increased risk of recurrent VTE is the early development of post-thrombotic manifestations (Stain et al. 2005).
3.3 Residual Vein Thrombosis
In a few cohort studies, the ultrasound persistence of residual thrombosis after an episode of proximal DVT was found to be an independent risk factor for recurrent VTE (Prandoni et al. 2002; Piovella et al. 2002) (Fig. 4). Two recent meta-analyses of available investigations suggest that, whichever the method used for measuring the thrombotic mass, residual vein thrombosis is a powerful and independent predictor of recurrent VTE (Tan et al. 2011; Donadini et al. 2014). Of interest, residual vein thrombosis predicts not only the development of recurrent ipsilateral DVT, but also that of contralateral DVT and even of PE apparently not associated with DVT, suggesting that residual vein thrombosis is a marker of hypercoagulability (Prandoni et al. 2002; Piovella et al. 2002; Tan et al. 2011; Donadini et al. 2014).
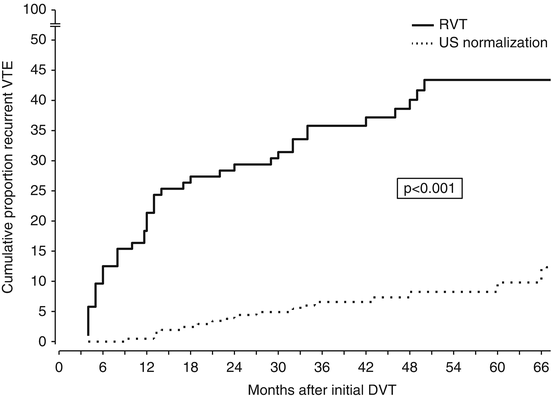

Fig. 4
Cumulative incidence of recurrent VTE according to the persistence of residual vein thrombosis (RVT) or ultrasonography normalization. The incidence of recurrent VTE after discontinuing anticoagulation is remarkably higher in patients with RVT than in those with earlier recanalization
In a recent prospective cohort study, residual vein thrombosis at 3 months after DVT (defined as the ultrasound incompressibility of at least 4 mm in the transverse section either in the popliteal or in the common femoral vein) was found to double the risk for subsequent recurrent VTE, post-thrombotic syndrome, arterial thrombotic events and cancer (Prandoni et al. 2015). The likelihood of residual vein thrombosis was higher in males, in patients with previous VTE and in those with extensive thrombosis. These findings suggest that a single assessment of residual thrombosis at 3 months can help risk stratify patients with proximal DVT and guide treatment decisions.
Whether the persistence of residual emboli after an episode of PE may predict the risk of recurrent PE as well is uncertain (Golpe et al. 2012). A recent prospective cohort study showed an unexpectedly high rate (85 %) of pulmonary artery recanalization in patients with PE treated with anticoagulants alone for 6 months, while in the remaining 15 % the thrombotic burden decreased by more than 80 % (Pesavento et al. 2014). These findings add to the current perception that residual thrombotic obstruction following PE is rare and unlikely to predict recurrent PE.
4 Risk Stratification Models
A novel approach for assessing risk of recurrent VTE consists of linking clinical patient characteristics with laboratory testing (Table 1). In a Canadian model, women with idiopathic VTE and none or one of several parameters (age older than 65, obesity, D-dimer positivity at time of discontinuing anticoagulation and post-thrombotic manifestations) exhibited a considerably lower risk of recurrent VTE than the remaining patients (Rodger et al. 2008). Another prediction model enables identification of the recurrence risk based on the combination of two baseline factors (sex and type of clinical presentation) and one post-baseline factor (D-dimer) (Eichinger et al. 2010). In the third model, based on a patient-level meta-analysis (the DASH score), D-dimer after stopping anticoagulation, age < 50 years, male sex and VTE not associated with hormonal therapy (in women) were found to be the main predictors of recurrence and were used to derive a prognostic recurrence score (Tosetto et al. 2012). All these scoring models require prospective validation before they can be applied in daily routine care (Kyrle and Eichinger 2012).
Table 1
Models to predict recurrent VTE
Men continue and HER D002 (Pesavento et al. 2014) | Vienna prediction model (Rodger et al. 2008) | DASH-score (Eichinger et al. 2010) | |
|---|---|---|---|
Study design | Prospective cohort | Prospective cohort | Patient level meta-analysis |
Patients | 646 | 929 | 1818 |
Predictive variables | Men: none | ||
Women: | |||
age ≥ 60 years | Sex | Abnormal D-dimer after anticoagulation | |
signs of PTS | Location of first VTE | Age < 50 years | |
BMI ≥ 30 kg/m2 | D-Dimer after anticoagulation | Male sex | |
D-dimer > 250 μg/l during anticoagulation | Hormonal therapy | ||
Increased risk of recurrent VTE | >1 point | >180 points (according to a nomogram) | >1 point |
Recurrence rate in patients at low risk | 1.6 % (95 % CI, 0.3–4.6) | 4.4 % (95 % CI, 2.7–6.2) | 3.1 % (95 % CI, 2.3–3.9) |
5 Duration of Treatment in Patients with Unprovoked VTE: Old and New Scenarios
Patients presenting with a first episode of unprovoked VTE should be offered at least 3 months of vitamin K antagonists, targeting an INR between 2.0 and 3.0 (Kearon et al. 2012), which is the duration that is commonly recommended for patients whose thrombotic episode is triggered by transient risk factors (Kearon et al. 2004). The decision as to go on or discontinue anticoagulation after the first 3 months should be individually tailored and balanced against the haemorrhagic risk, taking into account also patients’ preferences. An indefinite anticoagulation can be considered in selected patients at low bleeding risk (Kyrle and Eichinger 2012), while in all other patients this decision requires caution. Indeed, the annual incidence of major bleeding from long-term anticoagulation is 1.5–2.0 %, and the ‘case-fatality rate’ of major bleeding is considerably higher than that of recurrent VTE (Carrier et al. 2010; Lecumberri et al. 2013). Of interest, major bleeding increases the risk of recurrent VTE and decreases life expectancy (Spencer et al. 2008; Prandoni et al. 2010).
Following the demonstration that D-dimer can help stratify the individual risk of recurrent VTE (Palareti et al. 2002, 2003; Eichinger et al. 2003; Verhovsek et al. 2008; Bruinstroop et al. 2009), a randomized clinical trial showed that in patients in whom anticoagulation was withdrawn the annual rate of recurrent events was much lower in patients with negative (4.4 %) than in those with positive D-dimer (10.9 %), as assessed 1 month after warfarin discontinuation; however, it was higher than that (2.0 %) observed in patients in whom anticoagulation was not discontinued (Palareti et al. 2006). Of interest, in a recent prospective cohort study the annual rate of patients with unprovoked VTE who experienced recurrent events in the 2 years following the discontinuation of therapy owing to a negative D-dimer – as assessed 1 month after stopping warfarin – was found to be as high as 6.7 % (Kearon et al. 2015). Repeating D-dimer more times testing after anticoagulation suspension has the potential to identify individuals requiring resuming anticoagulation in order to prevent VTE recurrences (Cosmi et al. 2010).
In a few randomized clinical trials it has been shown that adjusting the duration of anticoagulation according to the persistence of residual thrombosis reduces the risk of recurrent VTE by approximately 40 % [(Prandoni et al. 2009; Siragusa et al. 2008)].
Of the two multicenter Italian studies, designed to evaluate the value of combining residual vein thrombosis with the serial D-dimer determination in adjusting the duration of anticoagulation, the DULCIS study has recently been published (Palareti et al. 2014). In this study, involving almost 1000 outpatients, D-dimer tests persistently below age and gender specific cut-offs were obtained in more than 50 % of patients, and discontinuation of vitamin K antagonists resulted in a subsequent annual recurrence rate lower than 3.0 %. The second – the Morgagni study – is still ongoing.
6 New Opportunities
6.1 New Oral Anticoagulants
New categories of oral antithrombotic drugs are emerging. These drugs have the potential to simplify the treatment of patients with VTE by obviating the need for periodic laboratory monitoring and are associated with a favourable benefit-to-risk ratio. They include compounds that selectively inhibit factor Xa, such as rivaroxaban, apixaban and edoxaban, and compounds that selectively inhibit thrombin, such as dabigatran etexilate. The results of several randomized clinical trials suggest that these drugs have a favourable benefit-to-risk profile in the first 6–12 months after an episode of VTE in comparison with conventional treatment (Schulman et al. 2009, 2014; The Einstein Investigators 2010, 2012; Agnelli et al. 2013a; The Hokusai-VTE Investigators 2013). The value of long-term administration in preventing recurrent VTE has been evaluated in four studies performed in patients who had completed a 6–18-month period of conventional anticoagulation.
Two clinical trials were designed to assess the efficacy and safety of dabigatran for the extended treatment of VTE. In the RE-SONATE study, 1343 patients with unprovoked VTE who had completed 6–18 months of anticoagulant therapy were randomized to dabigatran (150 mg bid) or placebo for an additional period of 6 months (Schulman et al. 2013). A 92 % relative reduction in the relative risk for recurrent VTE was shown in favor of dabigatran, with a low risk for major bleeding (0.3 % vs 0). In the RE-MEDY study, 2856 patients at a higher risk of recurrent VTE who had been treated for 6 months with vitamin K antagonists for a first VTE were randomized to dabigatran (150 mg bid) or warfarin (INR 2–3) for the long-term prevention of recurrent VTE (Schulman et al. 2013). Dabigatran was shown to be non-inferior to vitamin K antagonists (1.8 % primary outcome events in dabigatran patients versus 1.3 % in warfarin patients). Major bleeding complications occurred in 0.9 % and 1.8 % of patients, respectively (reduction in the relative risk, 48 %).
The EINSTEIN Extension trial randomized 1197 patients with unprovoked VTE who had completed 6–12 months of anticoagulant therapy for an acute index VTE event to an additional 6–12 months of therapy with either rivaroxaban, 20 mg once daily, or placebo (The Einstein Investigators 2010). Recurrent symptomatic VTE events were recorded in 1.3 % of patients in the rivaroxaban group and 7.1 % of patients in the placebo group (relative risk reduction, 82 %). Major bleeding occurred in 4 (0.7 %) rivaroxaban-treated patients as compared to none in the placebo group.
The AMPLIFY-Extension was a 12-month randomized clinical trial where apixaban 2.5 mg and 5 mg bid were compared with placebo for extended treatment to prevent recurrent VTE in approximately 2500 patients with unprovoked VTE who had completed 6–12 months of treatment for DVT or PE (Agnelli et al. 2013b). Apixaban demonstrated superiority versus placebo in the reduction of the composite endpoint of symptomatic, recurrent VTE and death from any cause (p < 0.001). Apixaban was superior to placebo for the predefined secondary efficacy outcome of recurrent VTE and VTE-related death (8.8 % in the placebo group, compared with 1.7 % in both the apixaban 2.5 mg and 5 mg groups; p < 0.001). The rates of major bleeding were comparable for 2.5 mg (0.2 %), 5 mg (0.1 %), and placebo (0.5 %) treatment groups. The rate of the composite of major bleeding and clinically relevant non-major bleeding for the 5 mg treatment group (4.3 %) was higher than in the placebo group (2.7 %), while 2.5 mg treatment group (3.2 %) demonstrated similar rates to placebo.
Because of the potential to induce fewer bleeding complications, the new drugs may open new scenarios for decisions on the optimal duration of anticoagulation in patients with unprovoked VTE. However, these findings should be interpreted with caution for a number of reasons. Only selected patients were included in the clinical trials, thus no information is available for different patients categories, including carriers of major thrombophilias, patients with severe renal or hepatic failure, patients requiring anticoagulation for reasons other than VTE and so on. In only one study (the REMEDY) was the new anticoagulant (dabigatran) compared with warfarin in patients at a higher recurrence risk (Schulman et al. 2013), whereas in all the remaining the comparator was placebo, and the extent by which recurrent VTE was reduced over placebo did not exceed that achieved by vitamin K antagonists in studies adopting a similar study design (Boutitie et al. 2011). In only one study (the AMPLIFY-Extension) was there a head-to-head comparison between the conventional and a lower dose of the index compound (apixaban), whereas in all the remaining the initial dose was tested unchanged throughout the whole period of anticoagulation (Agnelli et al. 2013b). As the duration of these studies did not surpass 6–12 months, the persistence of a favourable benefit-to-risk ratio beyond this period cannot be anticipated. The results of the AMPLIFY-Extension study suggest that halving the initial dose after the first 6 months in patients with unprovoked VTE is likely to preserve protection against recurrent VTE while further reducing the haemorrhagic risk (Agnelli et al. 2013b).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree