Ophthalmopathy
Petros Perros
Jane Dickinson
Ophthalmic abnormalities, better known as ophthalmopathy, are the principal extrathyroidal manifestations of Graves’ disease. The numerous synonyms for Graves’ ophthalmopathy (orbitopathy, thyroid eye disease) reflect its multifaceted clinical expression and the uncertainties about its pathogenesis, natural history, and treatment.
Epidemiology
Graves’ ophthalmopathy is clinically evident in about a third of patients with Graves’ disease, although it can be demonstrated by orbital imaging in nearly all (1,2,3). Its incidence seems to have declined in recent years for reasons that are unclear. Most patients have mild eye disease, while approximately 5% have severe potentially sight-threatening ophthalmopathy (2). The incidence of Graves’ ophthalmopathy in the United States, based on a population study performed many years ago, was 16/100,000 per annum for women and 2.9/100,000 per annum for men (4). Assuming a prevalence of Graves’ disease of approximately 1% in the general population (5) and that Graves’ ophthalmopathy is clinically apparent in 40% of patients with Graves’ disease, the prevalence of ophthalmopathy is 0.4%, or about 1.2 million in the United States, 3 million in Europe, and 27 million in the world. The age distribution in an incidence cohort shows two peaks: One at 40 to 44 years and a later one at 60 to 64 years for women, and 65 to 69 years for men (4). There is a female preponderance (77%) in patients attending specialist centers of approximately 2:1 (6).
Pathogenesis
Current evidence favors an autoimmune pathogenesis with important genetic and environmental influences, particularly smoking (2). Orbital muscle, connective tissue, and adipose
tissue are infiltrated by lymphocytes and macrophages (2). The extracellular compartment of extraocular muscles and orbital fibroadipose tissue becomes edematous, secondary to deposition of hydrophilic glycosaminoglycans, while the muscle cells themselves are unaffected (2,3).
tissue are infiltrated by lymphocytes and macrophages (2). The extracellular compartment of extraocular muscles and orbital fibroadipose tissue becomes edematous, secondary to deposition of hydrophilic glycosaminoglycans, while the muscle cells themselves are unaffected (2,3).
Anatomic Considerations
The orbit resembles a rigid cone with its base open anteriorly, where it is bounded by the semidistensible anterior orbital septum. Edematous expansion of orbital tissue has predictable consequences: Dysfunction of affected muscles, increased orbital pressure, and proptosis (exophthalmos, forward displacement of the eye). The clinical expression relating to each of these factors depends on the site and severity of inflammation and the potential for forward displacement. Several extraocular muscles, including the levator palpebrae superioris, are usually affected, although with variable frequency (2,3). This may lead to restriction of eye movements, lid lag, and incomplete eyelid closure. The latter may provoke sight-threatening corneal exposure, often compounded by proptosis. The degree of proptosis is limited by the length of the rectus muscles and the tightness of the anterior orbital septum. If the muscles are unable to stretch and the septum is tight, proptosis is minimal, but orbital pressure and venous congestion increase, and the rectus muscles may then compress the optic nerve at the orbital apex, with resulting visual loss. Conversely, if the septum is lax and the muscles are able to stretch, then proptosis increases, occasionally allowing subluxation of the eyeball. Acute inflammation may also cause erythema and swelling of the conjunctivae and eyelids, compounded by venous and lymphatic congestion. Muscle inflammation gives way to fatty degeneration and scarring, sometimes with further tethering and restriction. Various stages of these processes may coexist in one or more muscle tissues.
Immunology
The expansion of orbital tissues is primarily due to the edema that results from deposition of the very hydrophilic glycosaminoglycans. Orbital fibroblasts appear to synthesize and secrete these glycosaminoglycans in response to cytokines produced by infiltrating immune cells and macrophages and by the fibroblasts themselves (2,3). Cytokines also stimulate orbital fibroblasts, vascular endothelium, and macrophages to produce other immunomodulatory and inflammatory mediators. These in turn aid recruitment of T cells into the orbit and antigen recognition and presentation, and therefore perpetuate the local inflammatory response (2,3). A cell-mediated (Th1-type) immune response appears prominent early in the evolution of Graves’ ophthalmopathy, whereas humoral immunity (Th2-type), or both, may be relevant later in the course of the disease (2,3) and may be associated with fibrosis.
Evidence of autoimmune responses to thyroid antigens is invariably present in patients with Graves’ ophthalmopathy, including those with euthyroid Graves’ ophthalmopathy (2). An autoantigen shared by thyroid and orbit would explain the specific targeting of the latter and the close association between Graves’ thyroid disease and Graves’ eye disease. The most studied candidate autoantigen is the thyrotropin [thyroid-stimulating hormone (TSH)] receptor. This is expressed in orbital connective tissue, orbital fat (2,3), and extraocular muscle fibers (7), but at higher levels in patients with Graves’ ophthalmopathy than normal subjects (2,3). Orbital connective tissue contains adipocyte precursors (preadipocytes), which under appropriate conditions can differentiate into adipocytes (2,3). In vitro, expression of TSH receptors increases as fibroblasts differentiate to preadipocytes and adipocytes (2,3), and both differentiation and TSH-receptor expression are stimulated by cytokines (2,3). A central role for antibodies to the TSH receptor is also likely, by activating the fibroblast TSH receptor, leading to the differentiation of orbital fibroblasts into adipocytes (2,3), and thereby increasing orbital fat content (2,3). The absence of Graves’ orbitopathy in many patients with positive TSH-receptor antibodies suggests that other factors play a significant role in the pathogenesis of this disease. IGF-1 receptor expression in orbital fibroblasts from patients with Graves’ ophthalmopathy is increased compared to normal controls (7). Furthermore, the IGF-1 and TSH receptors appear to co-localize in orbital tissues from patients with Graves’ ophthalmopathy, and the two may form a functional complex (2). IGF-1 receptor expressing fibroblasts respond to IgGs from patients by producing chemoattractants (8). Circulating CD3 T cells from patients with Graves’ disease and some other autoimmune diseases also seem to express IGF-1 receptors (9). The role of these T cells in the pathogenesis of Graves’ ophthalmopathy is unknown but may support expansion of memory T cells. Circulating fibrocytes which express IGF-1 receptor and TSH receptor have been identified in much higher concentration in patients with Graves’ disease than in normal controls. (10). A concept is emerging that remodeling of connective tissue in the orbits and elsewhere may be driven by autoimmune processes and lead to the manifestations of Graves’ ophthalmopathy (3). IGF-1 and its receptor may represent an important switch, regulating the quality and amplitude of immune responses (2,3). The orbit may be constitutively primed to be metabolically more active than other tissues and therefore susceptible to inflammation (11). In the absence of a robust animal model, the precise roles of the TSH receptor, IGF-1 receptor, and effector cells remain at present elusive.
Genetic and Other Influences
The genetic contribution to Graves’ disease is substantial. The same risks and associations apply to Graves’ ophthalmopathy (12). Several studies examining genes that may distinguish between patients with Graves’ disease with and without eye disease have focused on associations between ophthalmopathy and alleles at the major histocompatibility complex (MHC), cytotoxic lymphocyte-associated antigen-4 (CTLA-4), and other loci (13). These studies have yielded contradictory results, which may be partly due to differences in allelotyping methodology, disease definition, race/ethnicity of the study subjects, and small sample sizes. Other factors that are associated with severe eye disease include advanced age, male sex, smoking, and persistent thyroid dysfunction, particularly hypothyroidism (2,3,12).
Natural History
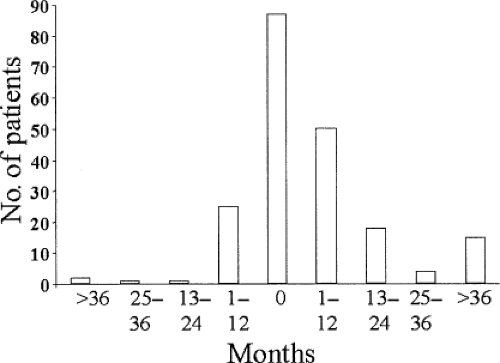
The onset of eye disease usually coincides with that of the thyrotoxicosis; however, exceptions are not uncommon, and
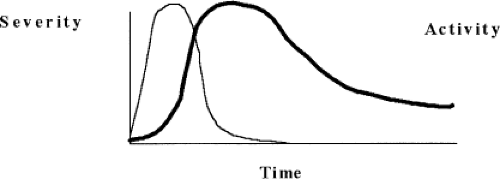
Graves’ ophthalmopathy can precede or follow thyrotoxicosis by months or even years (Fig. 18B.1). The severity of ophthalmopathy follows a phasic pattern: A phase of progressive deterioration lasting several months; a short period of peak severity; a phase of spontaneous improvement lasting up to a year or longer; and a quiescent phase when inflammatory signs disappear and clinical features stabilize, although they do not usually resolve completely. This pattern of change in severity over time was first described years ago (Fig. 18B.2) and has been confirmed by later studies (14). A related concept is disease activity, which relates to the presence of an acute inflammatory process within the orbit. Change in activity is implied by change in severity over time; its assessment is discussed later. Graves’ ophthalmopathy rarely becomes active again once it has become quiescent, but the activity and course may vary in individual eyes (15).
Graves’ ophthalmopathy can precede or follow thyrotoxicosis by months or even years (Fig. 18B.1). The severity of ophthalmopathy follows a phasic pattern: A phase of progressive deterioration lasting several months; a short period of peak severity; a phase of spontaneous improvement lasting up to a year or longer; and a quiescent phase when inflammatory signs disappear and clinical features stabilize, although they do not usually resolve completely. This pattern of change in severity over time was first described years ago (Fig. 18B.2) and has been confirmed by later studies (14). A related concept is disease activity, which relates to the presence of an acute inflammatory process within the orbit. Change in activity is implied by change in severity over time; its assessment is discussed later. Graves’ ophthalmopathy rarely becomes active again once it has become quiescent, but the activity and course may vary in individual eyes (15).
Clinical Presentation
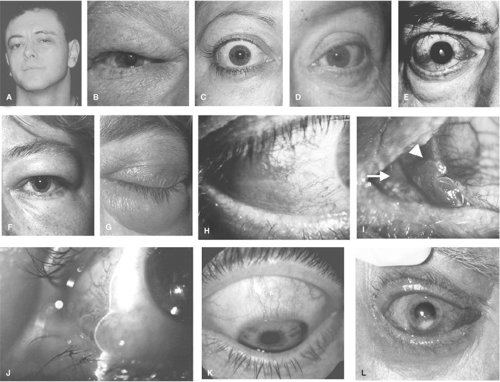
Graves’ ophthalmopathy is usually bilateral, but may be asymmetrical in 10% to 15% of cases (16) and occasionally remarkably unilateral, in which case the second orbit risks later relapse (17). The reason for asymmetry is unknown, though local anatomical and other factors may play a role. Most common presenting symptoms are of ocular surface discomfort and changes in appearance, particularly periorbital swelling (18) (Fig. 18B.3F). This usually develops over weeks to months, and although itching is absent, misdiagnosis as allergy is common. Other common symptoms are of retro-orbital pain, gaze provoked pain, and diplopia. Abrupt onset is less common but may herald a more severe course. Some patients describe slight diplopia as “blurring”; however, such blurring resolves with monocular occlusion. The myopathy of Graves’ ophthalmopathy relates to failure of relaxation in involved muscles, most commonly the levator palpebrae superioris and the inferior and medial recti. Upper-lid retraction affects 90% of patients at some stage (19), while rectus muscle involvement is demonstrable in the majority with asymmetry as the rule. Vertical strabismus is frequent, which may be accompanied by a compensatory head tilt (Fig. 18B.3A); however, many patients do not experience diplopia due to symmetrical inferior rectus involvement or because the restriction affects extremes of gaze irrelevant to daily life. Optic neuropathy is uncommon (less than 5%), but such patients may notice blurred vision unaffected by blinking or closing one eye, reduced color appreciation, or an awareness of gray areas of field loss. Older male smokers are most at risk of optic neuropathy with diabetes and vascular disease posing additional risks (20,21).
Most patients develop thyroid dysfunction some months before ophthalmopathy; in a minority they occur concurrently (Fig. 18B.1), and in 6% to 10% the characteristic ophthalmopathy presents without discernible thyroid dysfunction, termed euthyroid Graves’ ophthalmopathy or ophthalmic Graves’ disease. Most of these patients have onset of thyrotoxicosis within 18 months. About 5% of patients with ophthalmopathy have primary hypothyroidism rather than thyrotoxicosis: They tend to have milder, more asymmetrical ophthalmopathy (16). Concealed proptosis (Fig. 18B.3B) is a clinical variant in which ophthalmopathy is obscured by only minimal proptosis. It is not common, but is vital to recognize because of the high risk of optic neuropathy: These patients almost always have tense orbits and motility restriction, often with aching eye pain.
Severity and Activity of Eye Disease
Severity is defined as the degree of functional or cosmetic deficit at any time point in the course of the disease (22). Thus, variations in eyelid position, periorbital edema, proptosis, diplopia, corneal integrity, and optic nerve function are measures of severity. In contrast, activity implies the presence of acute inflammation and therefore potential for change either spontaneously or in response to medical treatment. Activity can be inferred directly when symptoms and signs of acute inflammation are present, or by demonstrating change in measures of severity via sequential assessments.
Clinical Assessment
The aim of initial assessment is to verify the diagnosis, ascertain severity, and determine the phase of the ophthalmopathy (Fig. 18B.2). A detailed assessment of symptoms (Table 18B.1) helps to highlight the patient’s concerns and priorities and informs mutual understanding and counseling. Ascertaining severity allows the clinician to determine the need for interventional treatment, while ascertaining activity and disease phase dictates the therapeutic options then available. Disease phase may be partly determined by inflammatory signs and any trend in symptoms, but reassessment after 2 to 3 months may be required.
Table 18B.1 Evaluation of Symptoms in Graves’ Ophthalmopathy | |
|---|---|
|
Table 18B.2 the Vancouver Orbitopathy Rule Consists of a Positive Response to Question 10 or 12 and One of Questions 7, 19, or 21 | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Diagnosis
Most symptoms and signs of Graves’ ophthalmopathy are individually nonspecific, reflecting periocular or orbital inflammation, muscle or nerve dysfunction, or volume change within the confined space of the orbit. Hence, other diagnoses should always be considered (23,24).
In patients with upper eyelid retraction (Fig. 18B.3C), Graves’ ophthalmopathy is likely if the patient has at least one of the following: Abnormal thyroid function, proptosis, optic neuropathy, or restrictive myopathy. If eyelid retraction is absent but thyroid dysfunction is present, the Vancouver Orbitopathy Rule may help to detect Graves’ ophthalmopathy (18) (Table 18B.2). Other highly suggestive signs include lid lag and a change in the contour of the upper eyelid known as lateral flare (Fig. 18B.3D). Typically, the combination of ophthalmic symptoms and signs plus recent or concurrent thyrotoxicosis will confirm the diagnosis without the need for supplementary studies (19). However, orbital imaging is highly recommended in patients with strictly unilateral signs, and a high index of suspicion for myasthenia or other pathology is needed for those without lid retraction or with divergent strabismus.
Evaluation of Disease Severity
Recommendations for objective assessment of Graves’ ophthalmopathy have been proposed (25), but there is no agreement as to precisely what to score and how to define it (22). A more detailed protocol with a soft-tissue atlas was published (22) and adopted by the European Group on Graves’ Ophthalmopathy (EUGOGO) (26); however, a further less detailed protocol has since been published in North America (27) and worldwide consensus remains elusive. Given the variable presentation of Graves’ ophthalmopathy, summary descriptions are of little use, and each feature should be assessed individually. The NOSPECS scheme (Table 18B.3) provides a useful mnemonic to assist this, although as a scoring system it has been justifiably criticized for poor definitions and reproducibility and for failing to record important change (22).
Assessment of Soft-tissue Changes
A complete examination protocol and photographic color atlas for the assessment of the soft-tissue changes that occur in patients with Graves’ ophthalmopathy can be found at http://www.eugogo.eu.
Eyelid and Periorbital Swelling
Orbital fat or lacrimal gland displacement can cause visible discrete eyelid swelling during any phase of ophthalmopathy (Fig. 18B.3E), whereas acute inflammation with sub-dermal fluid accumulation or tense skin thickening suggests active ophthalmopathy (Fig. 18B.3F). The edema of the eyelids, especially the lower lids, may be very marked (festoons) (Fig. 18B.3G), and this can persist long term. Like many soft-tissue features, lid swelling is best graded by photographic comparison to assess trend and treatment response (22).
Table 18B.3 Nospecs Classification of the Ocular Changes in Graves’ Ophthalmopathy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lid Erythema
Recent erythema of the eyelids implies active disease, but note that this sign may also persist.
Conjunctival Erythema
Erythema of the bulbar conjunctivae is associated with active disease (28,22) and resolves unrelated to eyelid retraction (Fig. 18B.3H).
Inflammation of the Caruncle or Plica
The caruncle is the triangular-shaped tissue inside the inner corner of the eyelids rich in sebaceous glands, while the plica is the vertical fold of conjunctiva just lateral to the caruncle. Inflammation in either of these structures suggests active ophthalmopathy (Fig. 18B.3H).
Chemosis
Chemosis denotes conjunctival edema (Fig. 18B.3J), which implies orbital congestion. In the acute situation, this is a surrogate marker of orbital inflammation.
Superior Limbic Keratoconjunctivitis
This describes inflammation of the upper bulbar and eyelid conjunctiva where they move over one another. It occurs in about 3% of patients with active ophthalmopathy (Fig. 18B.3K) and is associated with upper-lid retraction (29).
Palpebral Aperture
Widening of the palpebral fissure is usually due to retraction of both upper and lower eyelids and is caused by multiple factors (22). Upper-lid retraction probably relates mainly to levator and Müller’s muscle involvement plus tightness of the inferior rectus muscle, with smaller contributions from proptosis and scarring around the lacrimal gland fascia. Lower lid retraction correlates strongly with proptosis (Fig. 18B.3E). Standardized measurements require a consistent technique (distance fixation in a relaxed state, standardized head position, and, if vertical strabismus is present, occlusion of the contralateral eye). The width of the mid-pupil vertical fissure is measured, noting the distance between each lid and the adjacent limbus, plus the presence or absence of the upper-lid contour abnormality known as lateral flare (Fig. 18B.3D).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree