23 Oncologic emergencies
Metastatic spinal cord compression
Aetiology
The mechanisms by which MSCC may develop include:
Spinal cord metastases can be of three types depending on the location of tumour deposit:
Clinical features
Autonomic dysfunction is a late complication affecting up to 50% of patients with MSCC. It includes impotence, bladder and bowel dysfunction, with constipation occurring most frequently.
There are two clinical variants of MSCC, which require early recognition:
Investigations
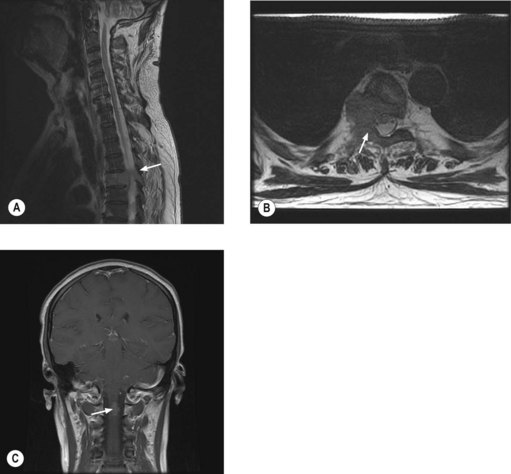
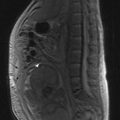
MRI of the whole spine is the investigation of choice for suspected MSCC. CT is used only when MRI is contraindicated. A sagittal screening of the whole spine with T1 and short T1 inversion recovery (STIR) images should be done to ensure that multiple levels of compression are not missed and also to identify asymptomatic metastases. T2 weighted images help to detect the degree of compression by a soft tissue component and intramedullary lesions (Figure 23.1).
Management
The aim of treatment is to preserve or improve neurological function and achieve pain control. 70–100% patients who are ambulant at the beginning of treatment remain ambulant with prompt treatment, whereas only 30–50% patients who are non-ambulant regain the ability to walk and only 5–10% of paraplegic patients become ambulant. Hence it is important to have definitive treatment within 24 hours of presentation with suspected cord compression. Patients with MSCC secondary to a vascular event will not respond to treatment.
Specific measures
Surgery may involve decompression, stabilization and or resection and reconstruction of the spinal canal. The patient’s overall prognosis and performance status should be taken into consideration and patients who have had no distal neurological function for >24 h should not be considered for surgery (Boxes 23.1 and 23.2).
Box 23.1
Patients with MSCC according to outcome after surgical intervention
| Good surgical candidates | Poor surgical candidates |
|---|---|
| ‘Good prognosis tumours’ e.g. breast cancer, testicular cancer etc. | ‘Poor prognosis tumours’ e.g. lung cancer, melanoma |
| Single level of compression with solitary or few vertebral metastases | Multiple levels affected or multiple spinal metastases |
| Absence of visceral metastases | Presence of visceral metastases |
| Good neurological function | Poor neurological function |
| No previous radiotherapy | Recurrence following radiotherapy |
| Minimal co-morbidity | Medically unfit for surgery |
| Unknown primary or no histopathological diagnosis | Prognosis <3 months |
Radiotherapy is the treatment most frequently used and is most effective for patients with radiosensitive tumours who are ambulatory at the beginning of treatment. For those without mechanical pain or structural instability, radiotherapy may significantly improve pain control and neurological function. The most commonly used regimes are 20 Gy in five fractions (more appropriate for patients with expected short survival) or 30 Gy in 10 fractions (Box 23.3). Some patients may deteriorate during radiotherapy when the steroid dose may be increased or they may be considered for surgery if appropriate. Patients with established paraplegia are treated with an 8 Gy single fraction for pain control.
Box 23.3
Radiotherapy in spinal cord compression
The issue of whether surgery or radiotherapy or a combination of both gives best functional outcome is yet to be resolved. A randomized study compared radiotherapy (30 Gy in 10 fractions) started within 24 hours of onset of MSCC with surgery within 24 hours followed by radiotherapy within 2 weeks of surgery. Results showed that initial surgery followed by radiotherapy offers a longer period with ability to walk compared with those treated with radiotherapy alone (median, 126 days vs. 35 days, p = 0.006). This study showed that surgery permits most patients to remain ambulatory and continent for the remainder of their lives, while patients treated with radiation alone spend approximately two-thirds of their remaining time unable to walk and incontinent. However, results of this study may not be extended to all patients as the study was limited to patients with less radiosensitive tumours and with different tumour types and presentations.
Prognosis
The median survival following a diagnosis of MSCC is 3–6 months, with 17% of patients alive at 1 year and 10% alive at 18 months. Pre-treatment motor function is an important predictor of the ambulatory outcome. Patients who develop motor dysfunction more slowly also may have a better outcome.
Paediatric spinal cord compression
Paediatric MSCC differs from adult MSCC in that it is often caused by chemosensitive histological types not seen in adults such as neuroblastoma, Wilms’ tumour (p. 323). The usual pathogenesis is the direct invasion of tumour through neural foramina. The most common histologic type is neuroblastoma, which responds to chemotherapy. Decompressive surgery is offered when patients present with rapid progression or progress during chemotherapy. Radiotherapy is only offered when there is no response after chemotherapy and/or surgery and for those who require palliation after failure of multiple systemic regimens.
Raised intracranial pressure
Approximately 25% of patients with cancer develop brain metastases and can present with features of raised intracranial pressure and focal neurological deficits. Seizures and bleeding into the metastases can result in an acute medical emergency. Malignant melanoma, renal cell carcinoma, thyroid cancer and choriocarcinoma are commonly associated with bleeding into the metastases. Initial treatment is dexamethasone 16 mg daily. Patients who present with seizures require anticonvulsants. Role of prophylactic anticonvulsants is not known; it may be considered in patients with high risk features of seizure such as metastasis to the motor cortex, bleeding metastasis and leptomeningeal metastasis. Further management of brain metastasis is given on p. 278.
Encephalopathy
Clinical features
Management
In patients with ifosfamide encephalopathy, treatment is based on the grade of the encephalopathy:
Febrile neutropenia
Introduction
Febrile neutropenia is one of the most common complications of cancer treatment. 50–60% of patients with febrile neutropenia have an established or occult infection and 20% of patients with a neutrophil count ≤1.0 × 109/l have bacteraemia.
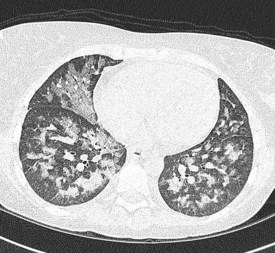
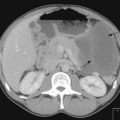
In the majority of cases, bacterial pathogens are responsible for febrile neutropenic episodes with fungal (Figure 23.3), viral and protozoal infections occurring more commonly as secondary events. Currently, Gram-positive bacteria account for 60–70% of microbiologically detected infections, which may in part be due to the prevalent use of quinolones as prophylactic antibiotics. Other possible causes of this change in trend include widespread use of intravenous catheters, along with more profound and prolonged neutropenia due to intensive and recurrent treatment regimes.