Common
Upon request
Special request
Rarely available
Solid cancers
Doxorubicin
Cisplatin
Folinic acid
5FU
Steroids
Dactinomycin
Methotrexate
Cyclophosphamide
Vincristine
Etoposide
Tamoxifen
LHRH/GnRH
Flutamide
Paclitaxel
Docetaxel
Capecitabine
Trastuzumab
Bevacizumab
Epirubicin
Aromatase inhibitors
Dacarbazine
Fludarabine
Stilbestrol
Bicalutamide
Mitoxantrone
Irinotecan
Oxaliplatin
Trastuzumab
Lapatinib
Temsirolimus
Carboplatin
Gemcitabine
Vinorelbine
Vinblastine
Gemcitabine
Ifosfamide
Estramustine
Abiraterone
Enzalutamide
Epothilones
Pertuzumab
Sorafenib
Cetuximab
Panitumumab
Temozolomide
Cabazitaxel
Erlotinib
Liposomal doxorubicin
Nab-paclitaxel
S1
Haematological cancers
Melphalan
Mercaptopurine
L-asparaginase
Hydroxyurea
Daunorubicin
Thioguanine
Bleomycin
Ara-C
Rituximab
Interferon
Carmustine
Idarubicin
ATRA
Procarbazine
Imatinib
Thalidomide
Interleukin
Gemtuzumab
Bendamustine
Lenalidomide
Bortezomib
Pomalidomide
Carfilzomib
Supportive medications
Metoclopramide
Prochlorperazines
Domperidone
Amoxicillin/clavulanic acid
Aminoglycosides
Fluconazole
Ketoconazole
Nystatin
Allopurinol
Zoledronic acid
Erythropoietin
Granisetron
Ondansetron
Lorazepam
Itraconazole
3rd-generation cephalosporins
Ibandronate
Vancomycin
Amphotericin B
4th-generation cephalosporins
Palonosetron
Aprepitant
Denosumab
Table 2
Drugs associated with major organ toxicities which require caution or monitoring
Renal dysfunction | Liver dysfunction | Cardiac dysfunction | Neuropathy |
|---|---|---|---|
Cisplatin Topotecan Capecitabine Ifosfamide Zoledronic acid | Ifosfamide Cyclophosphamides Temozolomide 5-Fluorouracil Gemcitabine Methotrexate Anthracyclines | Herceptin Anthracyclines Paclitaxel 5-Fluorouracil Capecitabine Mitoxantrone LHRH/GnRH Aromatase inhibitors | Paclitaxel Docetaxel Vinorelbine Platins Etoposide |
2 Non-Hodgkin’s Lymphomas
2.1 Follicular (Indolent Subtypes)
2.1.1 Chemotherapy
For follicular indolent types which are asymptomatic but advanced stage (stages III and IV) cases, watchful waiting can be applied. For symptomatic advanced stage patients, options are (a) single alkylating agent therapy such as chlorambucil 0.8 mg/kg orally every 3 weeks, cyclophosphamide at 1200–1500 mg intravenously every 3 weeks, 300–500 mg intravenously weekly or 60–100 mg orally daily and (b) combination chemotherapy such as chlorambucil, cyclophosphamide, vincristine, prednisone (LOP, COP), or doxorubicin added to the COP regimen (CHOP), or just an alkylating agent with prednisone, combination of fludarabine, mitoxantrone and high-dose dexamethasone (FND).
2.1.2 Targeted Therapy
Anti-CD20 monoclonal antibody (rituximab) in combination chemotherapy in indolent types of B-cell lymphoma or bortezomib, lenalidomide and bendamustine are useful with better overall survival compared to chemotherapy alone but are quite expensive.
2.2 Aggressive Subtypes
2.2.1 Chemotherapy and Targeted Therapy
Options include:
1.
Stage IA cases are preferably treated with limited chemotherapy, e.g. CHOP × 3 courses followed by involved-field irradiation.
2.
Advanced disease should be treated with aggressive combination chemotherapy till complete remission, possibly with a view to giving consolidating irradiation to areas of initially bulky disease.
CHOP remains the standard of care for most cases. The others include MACOP-B or hyper-CVAD with high-dose methotrexate and high-dose cytosine arabinoside when feasible.
For the B-cell-derived aggressive phenotype NHLs, R-CHOP is also not readily available in resource-poor settings; hence, CHOP remains the preferred treatment.
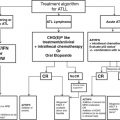
Precursor B-cell and precursor T-cell lymphoblastic leukaemia/lymphoma, Burkitt’ s lymphoma and some variants of diffuse large B-cell lymphoma and adult T-cell leukaemia/lymphoma (ATL) should be managed by physicians well versed with acute leukaemia therapy. The optimal treatment for peripheral, aggressive T-cell lymphomas excluding anaplastic large cell lymphoma is not clearly defined. CHOP is still the standard combination but with disappointing disease-free and overall survivals. Several other combinations have been tried with different results reported for various subcategories.
For CD30 expressing types, brentuximab in combination with combination chemotherapy appears promising.
B-cell subtypes associated with HIV/AIDS can be preferentially managed with concomitant rituximab, and chemotherapy is useful if affordable otherwise with chemotherapy alone.
3 Plasma Cell Myeloma
3.1 Treatment
1.
Supportive measures
2.
Specific antineoplastic agents
3.1.1 Supportive Measures
Supportive measures include correcting anaemia, pathological fractures (both established and impending), hypercalcemia, hyperuricemia, hyperviscosity, renal failure, dehydration and pain. The haemoglobin should be raised to 10 g/dl before starting specific anti-myeloma therapy, and likewise, any preexisting infection must be controlled. Bisphosphonates are recommended prophylactically in patients with significant myeloma bone disease.
3.1.2 Chemotherapy
Melphalan combined with prednisone is the most basic and effective treatment; however, new guidelines show additional benefit including thalidomide in the management of multiple myeloma as it is more efficacious compared to melphalan and prednisone alone. Because of their relatively low side effect profile especially to haematopoietic tissue, thalidomide and high-dose dexamethasone are recommended to be used up front in treatment of multiple myeloma. Thalidomide may be unavailable in certain situations. Other treatments include cyclophosphamide orally for 14 days followed by 14-day rest after which the same is repeated or intravenously 1,000 mg/m2 on day 1 plus prednisone 60 mg/m2/day on days 1–4 and a combination of vincristine and doxorubicin at 9 mg/m2/day, both by continuous infusion day 1–4 in combination with high intermediate doses of dexamethasone (VAD regimen). Oral idarubicin may be substituted for doxorubicin and appears to be equally effective and convenient.
3.1.3 Targeted Therapy
Where resources are available, targeted therapies include bortezomib in combination with doxorubicin and dexamethasone (VAD2); bortezomib and dexamethasone; bortezomib, melphalan, and prednisone (VMP); bortezomib, thalidomide and dexamethasone (VTD); or lenalidomide and dexamethasone. Lenalidomide is combined with liposomal doxorubicin.
More recent additions include carfilzomib, a novel proteasome inhibitor, and pomalidomide, a novel IMiD currently approved for third-line use.
3.1.4 Bone Marrow Transplant
This innovative method of treatment has been available for many years but is not accessible to many in LMIC except in academic institutions. Allogeneic haematopoietic progenitor cell transplantation is so far the only curative treatment for multiple myeloma, albeit in a very small minority. Autologous transplantation, especially of peripheral blood progenitor cells, is currently the standard of care for multiple myeloma. Melphalan should be avoided as much as possible in patients who are eligible for autologous transplant.
4 Hepatocellular Cancer
4.1 Chemotherapy
Most patients present with advanced tumours which can be treated with local ablative therapies including percutaneous chemoembolisation or ethanol embolisation, radiofrequency ablation or external beam irradiation. As a word of caution, HCC with attendant liver cirrhosis, especially Child-Pugh class C, has poor outcomes whatever the therapeutic intervention.
Various chemotherapeutic agents with modest influence on the course of the disease include doxorubicin, cisplatin, 5-fluorouracil and gemcitabine as single agents or in combination. The benefit versus the toxicity needs to be thoroughly discussed.
4.2 Targeted Therapy
Antiangiogenic agent bevacizumab has been used, with mixed and unimpressive results. Multikinase inhibitor, sorafenib, is the current standard of care, but again, its role is not universally accepted as it does not significantly improve disease-free or overall survival.
5 Breast Cancer
5.1 Hormone Therapy
Controversial reports of hormone receptor positivity rate in Africa are a limitation to the appropriate application of hormonal therapies in breast cancer management. Receptor testing is highly recommended where feasible as it gives important prognostic information. Receptor-positive patients invariably have high clinical response and survival rates with hormone therapies and poor clinical response to chemotherapies in general. Hormonal therapy is recommended as the first-line therapy for strong receptor-positive disease in the absence of severe symptomatic disease and is more acceptable to the patient. Even though hormonal therapy may be detrimental in hormone-negative subtypes, empirical use is unavoidable when patient does not respond to chemotherapy or cannot afford these medications. Aromatase inhibitors are considered more effective compared to tamoxifen in the postmenopausal setting however, without a survival advantage. Recent evidence points to increased efficacy when aromatase inhibitors are combined with ovarian suppression in the premenopausal state compared to tamoxifen with or without ovarian suppression. The high cost of aromatase inhibitors and the associated musculoskeletal side effects make it relatively unattractive leading to high rate of noncompliance. Tamoxifen is indicated in the pre- and postmenopausal woman and is readily available, relatively cheap and therefore highly recommended. Sequential treatment of aromatase inhibitors and tamoxifen may be another means of reducing overall cost without compromising effectiveness compared with tamoxifen alone in the postmenopausal woman.
5.2 Chemotherapy
The choice of chemotherapy depends of tumour biology, comorbidities, prior therapies and most importantly cost and convenience of administration. The cheapest and readily available regimens are the old regimens containing Adriamycin, 5FU, cyclophosphamide and methotrexate. They are still very effective in most breast cancer patients. Additional small but significant benefits are realised when Adriamycin in combination with taxanes is used for more aggressive-type tumours such as node-positive, triple-negative and her2-positive. Neoadjuvant chemotherapy is used frequently due to high representation of locally advanced cancers and long theatre waiting list. To date, there is no overall survival advantage compared to adjuvant therapies, and notably due to cultural influences, patients do not return for mastectomy especially following complete clinical responses. However, neoadjuvant chemotherapy approach may spare patients the use of medications that may not have worked. CMF is less commonly prescribed as it is considered suboptimal for aggressive tumour types. Classical CMF may have less compliance rates due to the multiple clinic visits and poor adherence with oral medication. Newer drugs such as gemcitabine, vinorelbine, capecitabine and epothilones used in combination with other drugs or as single agents have improved response and survival rates for recurrent and metastatic disease but are expensive and not readily available. Problems associated with weekly schedule drugs are neutropenia, cost of granulocyte colony-stimulating factors and inconvenience in administration, and therefore best administered in specialised centres. Triple-negative subgroups may have additional benefit if taxanes and/or platinum are included in first-line or subsequent lines of therapy, but CMF is an option when patient fails initial anthracyclines. Patients with metastatic disease can be managed with single agent sequentially without compromising overall survival which is less toxic, thereby reducing treatment-related morbidity and invariably a cost-saving measure. Clinical exam, tumour markers or radiological test where available should be used to access treatment response every 2–3 cycles and change regimen if there is no significant response.
5.3 Targeted Therapy
Advanced techniques for receptor testing are neither readily available nor affordable in low-resource settings. Lack of stringent quality assurance in the few testing laboratories has questioned the relevance of testing in such situations. Nevertheless, if done properly, it guides appropriate care. Her2-targeted therapies are expensive and not covered by national insurance policies. The benefits of her2/neu-targeted therapies where indicated are profound (up to 50 % reduction in recurrence rates and 37 % improvement survival). It is quite unfortunate that 20 % of women with breast cancer who are her2/neu positive in these parts of the world will not realise these benefits. Affordable generic substitutes should be widely available to these populations as a humanitarian gesture. The recommended 1-year duration of treatment is another limiting factor driving most oncologists to prescribe shorter regimes of 9 weeks to 6 months whose benefits even though inferior to longer treatment durations were better compared to those who did not receive her2-targeted therapy at all. Needless to say, second-line and dual targeting is completely beyond the scope of practice in most public institutions. Other therapies are being developed for triple-negative breast cancer but all at prohibitive cost. Tables 3, 4 and 5 summarise some common chemotherapy and hormonal and targeted therapies indicated for breast cancer management.
Table 3




Early breast cancer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree