Radionuclide
Half-life
Main emission
Radiopharmaceutical
Clinical use or relevance to thyroid nodules
123I
13 h
Gamma
[123I] NaI
Scintigraphy and iodine uptake
124I
4 d
Gamma and positron
[124I] NaI
PET imaging (thyroid cancer)
131I
8 d
Gamma and beta
[131I]NaI
Iodine uptake, therapy (beta emission), imaging thyroid cancer
99mTc
6 h
Gamma
99mTc pertechnetate, 99mTc MIBI
99mTc tetrofosmin
Scintigraphy (pertechnetate), incidental finding of nodules with high uptake (MIBI, tetrofosmin)
111In
67 h
Gamma
111In penetreotide
Incidental finding of nodule with high uptake
18F
110 min
Positron
18F FDG
18F-choline
Incidental finding of nodule with high uptake
11C
20 min
Positron
11C-choline
Incidental finding of nodule with high uptake
68Ga
68 min
Positron
68Ga SSTR (e.g., DOTATATE)
68Ga PSMA ligand
Incidental finding of nodule with high uptake
Iodine-131 (131I) for Uptake and Therapy
In nodular disease, 131I is only used for uptake measurements and therapy. A small amount of 131I in a form of sodium iodide (4–15 μCi/0.15–0.55 MBq) is typically given to measure uptake with a gamma probe [9]. 131I is used for whole-body imaging of thyroid cancer patients after thyroidectomy, but it should not be used for imaging of thyroid nodules [9]. 131I gives much higher radiation dose to the patient and to the thyroid tissue in particular, compared to 123I, and the image quality is inferior. Higher radiation dose is caused by long half-life of 131I (8 days) and its decay by β−-emission (606 keV) with 89% abundance and high-energy γ-emission (364 keV). However, β−-emission makes 131I very useful for therapy (see Section Selection of 131I activity for treatment of nodular disease for the activities recommended for therapy).
Iodine-123 for Imaging of Thyroid Nodules and Uptake Measurements
Iodine-123 (123I) in the form of sodium iodide ([123I] NaI) is the ideal radiopharmaceutical for thyroid imaging with gamma camera. It has favorable energy of gamma rays (159 keV) for imaging with gamma camera compared to 131I (364 keV). A half-life of 13 h is suitable for thyroid imaging, as compared to 8 days for 131I. Images can be done as early as 3–4 h after tracer administration. Usually the images are acquired at 24 h after administration of 123I, and uptake can be measured at the same time. Imaging of acceptable quality and uptake measurements can be performed up to 36 h after administration of 123I. It is given orally in administered activity of 200–400 μCi (7.4–14.8 MBq) [9].
99mTc Pertechnetate for Imaging of Thyroid Nodules and Uptake Measurements
Technetium-99 m (99mTc) pertechnetate is a negative ionic compound with similar distribution in the body as iodine. It is transported into the thyrocytes by the sodium iodide symporter. However, it cannot be organified in the thyroid tissue and quickly washes out. When 123I is not available, 99mTc pertechnetate can be used for thyroid imaging. 99mTc pertechnetate is eluted directly from a desktop generator and is thus much more easily available compared to 123I, and the cost is substantially lower. The procedure takes less time, but there is higher background uptake in other tissues at the time of imaging. 99mTc pertechnetate is less avidly concentrated in the thyroid, which results in lower target to background ratio. However, higher administered activity of 99mTc pertechnetate allows for comparable images, except when uptake is very low. 99mTc pertechnetate is given intravenously in administered activity of 2.0–10.0 mCi (74–370 MBq). Images are acquired 5–30 min after tracer administration.
Iodine-124 for Thyroid Imaging and Uptake
The positron-emitting radioisotope of iodine, 124I (positron abundance 23%), has been used in research studies for thyroid imaging and radioiodine uptake measurements [10–12]. PET/CT scanners have much better image (spatial) resolution than conventional gamma cameras (~4 mm versus ~10 mm). PET/CT with 124I allows for more precise measurements of tracer uptake compared to planar or SPECT/CT studies; thus radioiodine imaging with PET/CT is an attractive idea. Poor availability, lack of FDA approval, high cost, and lack of reimbursement of 124I make it not yet practical to use. 124I PET/CT has mainly been studied for staging and dosimetry before radioiodine treatment in differentiated thyroid cancer [13].
Thyroid Nuclear Imaging Procedures
Thyroid imaging is typically performed with a gamma camera with a pinhole collimator, and the imaging is often called “thyroid scintigraphy ” or “thyroid scan” (Fig. 4.1). The pinhole collimator will magnify the gland when the camera is close to the organ. It allows for better image resolution compared to standard parallel-hole collimator at the expense of some geometric distortion of structures located at different depths. Typically, anterior images with markers on the thyroid cartilage and suprasternal notch, as well as bilateral anterior oblique images, are acquired. Correlation between a palpable nodule and a scintigraphic nodule can be done by placing a radioactive marker on the nodule or even “drawing a contour” on the image with a radioactive marker [14]. SPECT/CT may be used as a supplement to planar imaging (Figs. 4.2 and 4.3) for better anatomical correlation with nodules detected on ultrasound, for volume measurements, or for localization of ectopic thyroid tissue. Much better anatomical information on SPECT/CT allows for better correlation between ultrasound and scintigraphic finding.
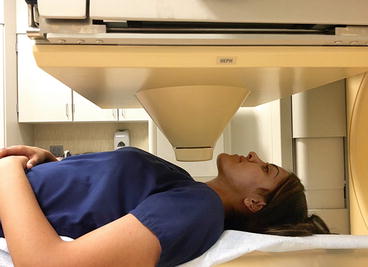
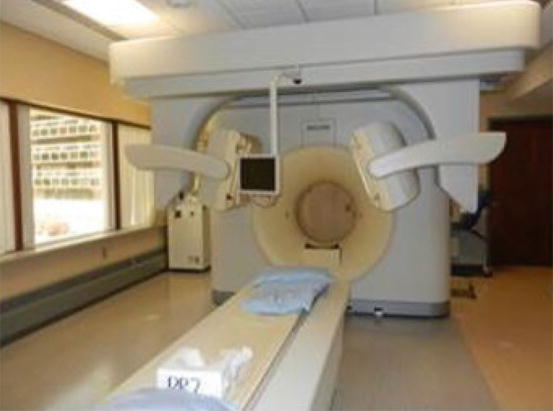
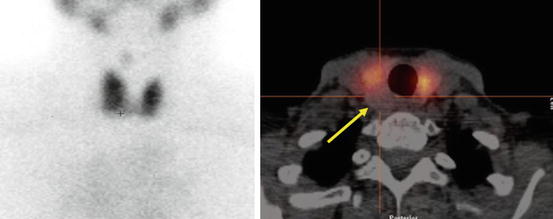

Fig. 4.1
Gamma camera with pinhole collimator for thyroid imaging

Fig. 4.2
SPECT/CT may be used for better anatomical correlation of nodules with better correlation or to localize ectopic thyroid tissue. It allows for attenuation correction and volume measurements

Fig. 4.3
“Cold” nodule on thyroid scintigraphy having benign cytology. Patient with a 1.2 cm thyroid nodule posterior in the right lobe found incidentally on CT. A 99mTc pertechnetate scan was performed before US-guided FNC. Left: Planar scan. No “node,” neither “cold” nor “warm,” can be seen. Right: SPECT/CT transaxial slice shows that the thyroid nodule is “cold” (arrow). US-guided FNC was performed and cytology showed benign cells. This case is an example of the usefulness of SPECT/CT supplemental to planar imaging
Interpretation of Thyroid Scintigraphy
Thyroid scintigraphy is evaluated for the presence of “hot” (tracer uptake greater than the surrounding normal thyroid uptake), “warm” (uptake equal to adjacent thyroid tissue), or “cold” uptake (less than the surrounding thyroid tissue) areas that correspond to palpable nodules or to nodules seen on US.
“Hot” Thyroid Nodules
“Hot” nodules may be solitary or a part of multinodular disease (Fig. 4.4). Occasionally a “hot” nodule may represent normal thyroid tissue surrounded by an area of decreased uptake; however, most “hot” nodules represent follicular adenoma, oncocytic (Hürthle cell) adenomas, or hyperplastic (adenomatous) nodules. They may demonstrate a central area of decreased uptake due to cystic degeneration as will be seen on US. “Hot” nodules usually function independently of TSH stimulation. They may produce sufficient amount of hormones to cause hyperthyroidism and suppress TSH and suppress uptake in normal thyroid tissue, with only the nodule visible on the scan (Fig. 4.5). Autonomous nodule was originally defined as a nodule that does not decrease uptake after administration of thyroid hormones [15]. Thyroid suppression test is not performed in current US practice, and the terms “hot” nodule and autonomous nodule are often used interchangeably. Suppression test is, however, still used in some European practices (per personal communication). Not all patients with “hot” nodules are hyperthyroid, especially in iodine-deficient areas. A European study demonstrated that 49% of 368 patients with autonomous functioning nodules had normal TSH [16]. Cytology from a hyperfunctioning nodule, typically indicating follicular neoplasia, could lead to unnecessary hemithyroidectomy, so scintigraphy is often used, outside of US, to guide FNA from a nodular goiter.
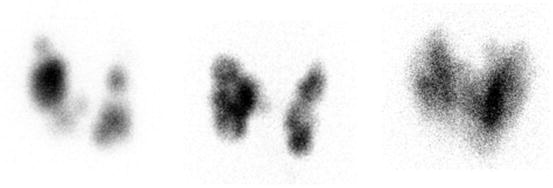
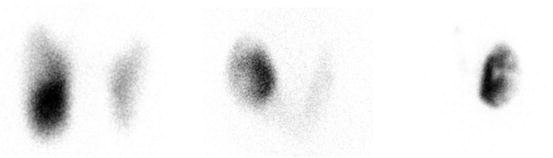

Fig. 4.4
Three patients with toxic multinodular goiter . Left: F 31 y. 123I uptake 34% at 24 h. Treated with surgery because of young age. Middle: F 83 y. 123I uptake 25% at 24 h. Treated with 45 mCi (1.7 GBq) of radioiodine. Right: F 66 y. 123I uptake 23% at 24 h. Treated with 15 mCi (555 MBq) of radioiodine

Fig. 4.5
Three examples of autonomous nodule with various degrees of suppression of normal thyroid tissue
Malignancy in a “hot” nodule is rare. The 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer (2015 ATA Guidelines) state: “Since hyperfunctioning nodules rarely harbor malignancy, if one is found that corresponds to the nodule in question, no cytological evaluation is necessary” [17]. However, malignancy can occasionally be present in a “hot” nodule [18]. A literature search of surgical patients with solitary functioning nodules, managed by thyroid resection, revealed an estimated 3.1% prevalence of malignancy [19]. Of 77 reported cases, 57.1% had papillary carcinoma, 36.4% had follicular thyroid carcinoma (FTC) , and 7.8% had Hürthle cell variant of FTC (HTC) (FTC and HTC were more frequent than usual). Compared to individuals with benign hyperfunctioning thyroid nodules, those with malignant hyperfunctioning nodules were younger and more predominantly female.
“Cold” Thyroid Nodules (Fig. 4.6)
The risk of malignancy in “cold” nodules varies between different reports but has been reported as high as 25% [20]. A study of 5637 patients with “cold” nodules demonstrated an overall 4.6% frequency of malignancy [21]. Frequency was higher in iodine-sufficient areas (5.3%) versus iodine-deficient areas (2.7%). The frequency of cancer was significantly lower in female patients with “cold” nodules (4.2%) than in males (8.2%). The proportion of nodules that were malignant was smallest in patients of the fourth decade and was greatest in patients younger than 30 years or older than 60 years. Finally, the frequency of thyroid cancer in patients with a solitary nodule was not different from the frequency in patients with multiple nodules.
In current US practice, where only patients with subnormal TSH undergo thyroid scintigraphy, “cold” nodules are seen much less frequently than in the past, usually in patients with toxic multinodular goiter or patients with Graves’ disease and a coexisting nodular disease [22] (Fig. 4.6). Solitary “cold” nodules or dominant “cold” nodules in a multinodular goiter are evaluated with US and US-guided FNA to exclude malignancy prior to radioiodine therapy for hyperthyroidism.
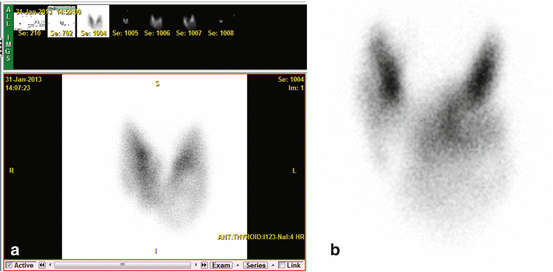

Fig. 4.6
Two hyperthyroid patients with cold nodules . (a) 123I uptake 45%. FNA suspicious for follicular neoplasm. Pathology after resection: minimally invasive follicular carcinoma. (b) Patient with history of Graves’ disease . Fine needle cytology showed a benign lesion. 123I uptake 25%. Treated with 15 mCi (555 MBq) of radioiodine for Graves’ disease
It is important to exclude malignancy in a patient with Graves’ disease and “cold” nodule, as patients with Graves’ disease tend to have more aggressive thyroid cancer [23]. A European multicenter study of 140 patients with coexistent Graves’ disease and thyroid nodules, treated with surgery, showed a 15% rate of malignancy [24]. Another study of 60 patients with Graves’ disease and nodules showed 10% of malignancy [22].
Simultaneous existence of autonomous nodules and Graves’ disease (Marine-Lenhart syndrome ) occurs in 0.8–2.7% among patients with Graves’ disease [22, 25, 26]. Even though these nodules are radioiodine avid, they sometimes accumulate relatively less iodine than the remainder of the gland involved with Graves’ disease. They may appear either “cold” or “hot” on the thyroid scan [26]. Radioiodine treatment of these patients may require higher administered activity than used for Graves’ disease [26]. There is at least one case report of malignancy in Marine-Lenhart syndrome [27].
Interpretation of Thyroid Scan with 99mTc Pertechnetate vs. 123I for Imaging of Thyroid Nodules
Since 99mTc pertechnetate is not handled by the same physiologic mechanism as iodine, 99mTc pertechnetate scan findings in thyroid nodules may occasionally be discordant from radioiodine imaging findings. The nodules with 99mTc pertechnetate uptake, but no radioiodine uptake at 24 h, are believed to carry higher risk of malignancy, as the thyroid cancer cells often retain the ability to concentrate iodide without incorporating it into the thyroid hormones. However, no malignancies were found in discordant nodules in a study of over 300 patients with a solitary or dominant palpable nodule, imaged both with 99mTc pertechnetate and 123I NaI [28]. Discrepancies were found in 5–8% of cases, twice as often in multinodular goiters as in single nodules. There was no correlation between the histopathology and the type of discrepancy. Nodules with higher 99mTc pertechnetate uptake than 123I uptake were more common than the reverse. Twelve carcinomas were found (4%), but none in the nodules showing discrepancy. However, Reschini et al. found two malignancies in seven patients, who had nodules with 99mTc pertechnetate uptake at 30 min, but no 24 h radioiodine uptake. It was a prospective study of 140 patients with hot or warm nodules on 99mTc pertechnetate scan and normal TSH [29].
Measurement of Uptake of Radiopharmaceuticals in the Thyroid Gland
123I and 131I Radioiodine Uptake Measurement
Radioiodine uptake measurement is performed in nodular thyroid disease to help select the amount of 131I activity needed for radioiodine therapy. In some cases, low radioiodine uptake may indicate that the radioiodine therapy would not be indicated, for example, when hyperthyroidism is a result of a thyroiditis coexisting with nodular goiter (Fig. 4.7).


Fig. 4.7
Hyperthyroidism . Nodular gland. Neck discomfort and pain on palpation. 123I uptake 0.5% at 4 h (normal in our institution 3–16%). Diagnosis: subacute thyroiditis. Hyperthyroidism resolved on follow-up. Biopsy of dominant cold nodule was benign
Radioiodine uptake in the thyroid gland of patients with hyperthyroidism caused by nodular disease is often only mildly elevated (lower than typically observed in Graves’ disease) and sometimes at the upper range of normal. Normal radioiodine uptake range in the USA is 10–30% at 24 h after administration of radiopharmaceutical. Upper limit of normal uptake is typically higher in areas of iodine deficiency and may be up to 50% [30]. At Mayo Clinic, Rochester, USA, we sometimes do a 4–6 h uptake measurement and extrapolate the 24 h uptake value using the following formula suggested by Hayes et al. [31]:
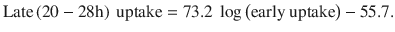
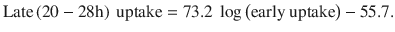
However, this formula is probably not as accurate for patients with nodular thyroid disease, because Hayes et al. did their study on patients with Graves’ disease . When 123I is used for imaging, uptake measurement is done at the same time, typically at 24 h after administration.
Procedure for Uptake Measurement
Radioiodine uptake in the thyroid gland can be measured using a thyroid uptake probe or a gamma camera. When thyroid probe is used (Fig. 4.8), counts are typically measured over the patient’s neck and over the patient’s mid-thigh at the same distance and for the same length of time. A source of the same radionuclide of identical activity as given to the patient is placed in a standard neck phantom and measured for the same time with the same geometry, followed by room background activity measurement for the same time. Thyroid uptake is calculated using the following formula:


Fig. 4.8
Thyroid probe for uptake measurements
%Uptake = [(neck counts − thigh counts)/(standard counts – room background counts)] × 100.
When a gamma camera is used for uptake measurement, a known small amount of the tracer is placed in a neck phantom and positioned beside the head of the patient, in the field of view of gamma camera with a parallel-hole collimator (Fig. 4.9). In this method, administered activity has to be corrected for decay during the time elapsed since administration.
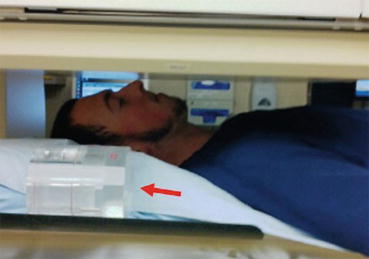

Fig. 4.9
Gamma camera with parallel-hole collimator for imaging and uptake measurements. Neck phantom with standard activity in the field of view (arrow)
99mTc Pertechnetate for Thyroid Uptake Measurement
When 99mTc is used for imaging, a small amount of 131I in a form of sodium iodide is typically used to measure uptake with a gamma probe. 99mTc pertechnetate uptake can be used in lieu of radioiodine uptake [32]. It is sometimes used in Europe, but not in the USA. A formula to estimate 24 h radioiodine uptake using a 5-min 99mTc sodium pertechnetate uptake has been published [33]:
![$$ 24\mathrm{h}\ \mathrm{Iodide}\ \mathrm{Uptake}=\left[17.72\times \mathrm{In}\left(5{\mathrm{minute}}^{99\mathrm{m}}\mathrm{Tc}\ \mathrm{pertechnetate}\ \mathrm{Uptake}\right)\right]+\mathrm{30.40.} $$](http://oncohemakey.com/wp-content/uploads/2019/09/A418021_1_En_4_Chapter_Equb-1.gif)
![$$ 24\mathrm{h}\ \mathrm{Iodide}\ \mathrm{Uptake}=\left[17.72\times \mathrm{In}\left(5{\mathrm{minute}}^{99\mathrm{m}}\mathrm{Tc}\ \mathrm{pertechnetate}\ \mathrm{Uptake}\right)\right]+\mathrm{30.40.} $$](http://oncohemakey.com/wp-content/uploads/2019/09/A418021_1_En_4_Chapter_Equb-1.gif)
Patient Preparation for Thyroid Scintigraphy and Radioiodine Treatment
Thyroid medications and iodine-containing products, including iodine-based contrast agents, will interfere with thyroid uptake and should be discontinued or avoided prior to thyroid scintigraphy and radioiodine therapy (Table 4.2).
Lactating mothers have to refrain from breastfeeding for 3 days after 123I administration [35] and for 12–24 h after 99mTc pertechnetate injection [35, 36]. Breast milk should be expressed and discarded during the interruption period. Breastfeeding cannot be resumed after administration of 131I [36, 37].
Thyroid scintigraphy, uptake measurements, and radioiodine therapy are all contraindicated during pregnancy.
Table 4.2
Thyroid medications and iodide-containing products that will interfere with thyroid uptake and need to be discontinued prior to thyroid scintigraphy and radioiodine therapy (adapted from 2012 SNMMI Procedure Standard)
Type of medication | Recommended time of withdrawal |
|---|---|
Water-soluble intravenous radiographic contrast agents | 6–8 wka, assuming normal renal function |
Lipophilic intravenous radiographic contrast agents | 1–6 mob |
Thyroxine | 3–4 wk |
Triiodothyronine | 10–14 dc |
Antithyroid drugs (methimazole) | 5–7 d |
Nutrition supplements containing iodide | 7–10 d |
Kelp, agar, carrageenan, Lugol solution | 2–3 wk, depending on iodide content |
Saturated solution of potassium iodide | 2–3 wk |
Topical iodine (e.g., surgical skin preparation) | 2–3 wk |
Amiodarone | 3–6 mo or longer |
Additional preparations prior to radioiodine therapy:

Radioiodine therapy has to be postponed for at least 6 weeks after cessation of breastfeeding, to allow the hypertrophied breast tissue to involute and avoid unnecessary radiation dose to the breasts. Treatment with lactation-inhibiting medications, such as bromocriptine or cabergoline, has been shown to reduce breast uptake [38].
Pregnancy test is recommended in all female patients who have a potential to be pregnant, ideally within 24 h prior to radioiodine. Preferably a blood test should be performed, as it is more sensitive than urine test. In addition to a pregnancy test, it has to be assured that a female patient is on adequate contraception. Pregnancy test may remain negative up to 7–10 days after fertilization, so it is best to postpone therapy until the beginning of the next menstrual cycle in patients who may have had unprotected intercourse in the 10 days before treatment. Prior hysterectomy has to be well documented before omitting the pregnancy test. Some institutions perform pregnancy test even if patient has a history of tubal ligation.
It is usually recommended to avoid getting pregnant for 6 months after radioiodine therapy.
Patients should be instructed on the therapeutic alternatives, risks, need for follow-up tests, potential hormone supplementation, and a potential for re-treatment.
Patients have to be asked about urinary incontinence (to limit contamination with radioiodine), nausea, and possibility of vomiting.
Treating physician has to make sure that the patient is able to follow radiation safety instructions.
Radiation safety precautions vary depending on the local regulations. SNMMI and ATA have published their recommendations [8, 39].
SNMMI Guidelines for patients receiving radioiodine treatment are shown in Fig. 4.10 .

Fig. 4.10
SNMMI Guidelines for patients receiving radioiodine 131I treatment
Radioiodine Therapy of Benign Thyroid Nodules
Thyroid nodular disease causing clinical or subclinical hyperthyroidism is usually treated with 131I or surgery. The decision is based on multiple factors including patient’s age, comorbidities, personal preference, size of goiter, radioiodine uptake, need for rapid correction of hyperthyroidism, history of prior surgery, local expertise, and availability [40]. The degree of reduction of goiter size after radioiodine treatment varies. In a prospective study of 62 patients with solitary toxic thyroid nodules by Nygaard et al., thyroid volume was reduced by 35% within 3 months after radioiodine therapy and by 45% after 24 months [41]. In a European study of 438 patients with borderline hyperthyroidism and multifocal and disseminated autonomy, decrease in thyroid volume ranged between 10 and 60% with a mean reduction of 37% [42].
Toxic Multinodular Goiter
For toxic multinodular goiter , the risk of subsequent hypothyroidism is much lower with radioiodine therapy compared to surgery. A study from Mayo Clinic reviewed records of 253 patients with toxic multinodular goiter treated with an average of 30 mCi (1.1 GBq) of 131I, ranging from 10 to 100 mCi (370 MBq–3.7 GBq) between 1975 and 1993 [43]. Patients had a 28% risk of becoming hypothyroid at 1 year after therapy, compared to 89% of patients treated with surgery. Patients had an estimated probability of radioiodine treatment success of about 90% at 2 years after therapy. Cost of radioiodine therapy is much lower than surgery, and side effects and morbidity are minimal. Patients with toxic multinodular goiter tend to be older with potentially higher immediate risks of surgery. However, surgery is preferred when the goiter is very large and would require high activity of radioiodine or if posttreatment swelling of the goiter could potentially affect adjacent structures. Surgery is also preferred if rapid control of hyperthyroidism or thyroid size is required. It has an advantage of removing incidental foci of malignancy.
Radioiodine Treatment of Solitary Toxic Nodules
For solitary toxic nodules , the choice between radioiodine and surgery is less clear. Unilateral procedure has less surgical risks and much less risk of hypothyroidism compared to total thyroidectomy. For example, in a retrospective study of 630 thyrotoxic patients treated with surgery, 35 patients with solitary toxic adenoma who underwent lobectomy had a 14% incidence of hypothyroidism [44].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree