Syndrome
Gene
Chromosomal location
Inheritance
Type of cancer
Other features
Isolated FMTC [151]
RET
10q11.2
AD
MTC
MEN2A [151]
RET
10q11.2
AD
MTC
Pheochromocytoma, hyperparathyroidism
MEN2B [151]
RET
10q11.2
AD
MTC
Pheochromocytoma, neuromas and ganglioneuromas, marfanoid habitus
Isolated FNMTC [152]
2q21
PTC
DICER 1 [153]
DICER 1
14q32.13
AD
PTC (only seen after chemotherapy)
Multinodular goiter, pleuropulmonary blastoma, Sertoli-Leydig tumors of the ovaries
Pendred syndrome [154]
SLC26A4
7q31
AR
FTC/PTC
Goiter, hypothyroidism, sensorineural hearing loss
Carney complex [155]
PPRKAR1a
17q23-24
(CNC1 locus)
2p16
(CNC2 locus)
AD
FTC/PTC
Pigmented lesions of the skin and mucosa; cardiac, cutaneous and other myxomatous tumors
Werner syndrome [156]
WRN
8p11-12
AR
FTC/PTC/ATC
A progeroid syndrome: cataracts, thinning of the scalp, premature gray hair and short stature, diabetes mellitus, hypogonadism, osteoporosis, premature atherosclerosis
Intestinal polyposis syndromes: [157]
Familial adenomatous polyposis [157]
APC
5q21
AD
FTC/PTC
Multiple colorectal adenomas, hepatoblastoma, medulloblastoma
Gardner’s syndrome
APC
5q21
AD
FTC/PTC
In addition to FAP features: desmoid tumors, sebaceous or epidermoid cysts, lipomas, osteomas, fibromas, supernumerary teeth, juvenile nasopharyngeal angiofibromas, and adrenal adenomas
Peutz-Jeghers syndrome [158]
STK11
19p13.3
AD
PTC
Pigmented mucocutaneous macules and multiple hamartomatous gastrointestinal polyps
PTEN hamartoma syndromes:
Cowden disease [159]
PTEN
10q23.2
AD
FTC/PTC
Hamartomatous tumors, trichilemmomas, acral keratosis, facial papules and oral papillomas, developmental delay, breast cancer
PTEN
10q23.2
AD
FTC/PTC
Hamartomatous tumors, macrocephaly, penile lentigines, developmental delay, myopathy
Evaluation of a Thyroid Nodule
The evaluation of a thyroid nodule consists of a history, physical examination, thyroid ultrasound, lab evaluation, potentially fine needle aspiration biopsy, and possibly thyroid scintigraphy. The current ATA recommendations are outlined in Fig. 14.1. When a child or adolescent presents for evaluation of a thyroid nodule which they have noticed themselves or which was picked up incidentally on physical examination or imaging, the history should ascertain if there is an increased risk of cancer: thyroid cancer is typically painless and not associated with any inflammation of the neck. The vast majority of patients with thyroid cancer are euthyroid. A prior history of radiation exposure or a family history of thyroid cancer should be sought. The physical exam should include palpation of the thyroid gland and the neck to determine the presence or absence of concerning lymphadenopathy. Although the hereditary syndromes described in Table 14.1 are rare, they need to be kept in mind when performing the physical examination, e.g., abnormal pigmentation in Carney complex (see Fig. 14.2) and Peutz-Jeghers syndrome and hamartomatous lesions in the PTEN hamartoma syndromes. It must also be borne in mind that several non-thyroidal conditions, including abscesses, lymphatic or vascular malformations, ectopic thymus, or thyroglossal duct cysts, can mimic thyroid nodules. Nodular disease of the thyroid may be due to a solitary thyroid nodule, multinodular goiter, or nodules in a goiter due to chronic lymphocytic thyroiditis, or it may be non-palpable found incidentally.
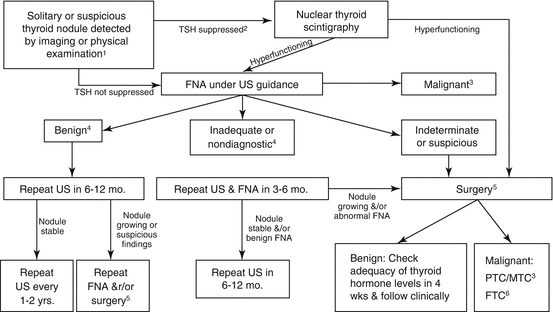
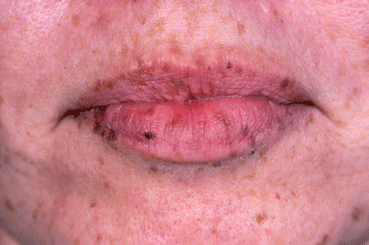

Fig. 14.1
Algorithm for the evaluation and treatment of thyroid nodules in children per the 2015 ATA guidelines (Republished with permission from Mary Ann Liebert, Inc. [71]). 1Assumes a nodule with suspicious US features in a patient without risk factors for thyroid malignancy. 2Free T4 may be normal or low. 3Bethesda category VI on cytology, requires total thyroidectomy and need or extent of neck dissection depends on extent of disease (extrathyroidal spread/cervical lymph node involvement). 4Bethesda Category I and II, surgery can be considered for compressive symptoms, if US features are suspicious, lesion is >4 cm, or family is uncomfortable with ambiguity of diagnosis and close follow-up. 5Surgery should consist of at least lobectomy with intra-operative frozen section and completion thyroidectomy if differentiated thyroid cancer is diagnosed. 6Consider completion thyroidectomy, may require radioactive iodine therapy

Fig. 14.2
Abnormal pigmentation of the lips seen in Carney complex (Photo courtesy of Dr. J. Aidan Carney, MD, PhD)
Thyroid Ultrasound
Thyroid ultrasound (US) is the best imaging modality for thyroid nodules. Prior guidelines have recommended against performing a biopsy on any nodules <1 cm unless the patient is at high risk (prior radiation exposure, at-risk genetic syndrome, or pathologic-appearing regional lymph nodes) [13]. In adults, there is growing evidence to support this clinical approach, given the current knowledge regarding the indolent nature of papillary thyroid microcarcinoma (PTMC) (PTC measuring <1 cm); only 3.5% of them grow in adults [64].
The thyroid gland is smaller in children than in adults, and therefore, a tumor <1 cm in a child and in an adult may not be comparable. Thyroid cancer measuring <1 cm is less common in children, and the term PTMC is not typically used. The frequency of papillary thyroid carcinomas in children and adolescents measuring less than 1 cm ranges from 1 to 37% in various studies, and it increases with age, even within the pediatric age ranges [14, 65–70]; when present, it may not be as indolent as in adults. Children with PTC ≤1 cm have more frequent lymph node metastases than adults with PMTC of 66.7% (12/18) in one study [69] and 41% (14/34) compared to 19% of adults (85/584) in another study which included adults and children [70].
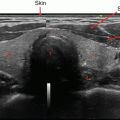
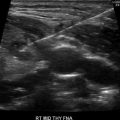
The most recent pediatric guidelines from the American Thyroid Association [71] have recommended proceeding with biopsy in nodules of any size which have suspicious US characteristics. Hypoechogenicity, irregular margins, increased blood flow, the presence of microcalcifications, and abnormal cervical lymph nodes are all more common in malignancy, and their presence should prompt consideration of biopsy [72, 73] (see Fig. 14.3). A recent meta-analysis and systematic review has shown that the presence of internal calcifications or enlarged cervical lymph nodes were the US features with the highest likelihood ratio (4:46 and 4:96, respectively) for thyroid cancer. A cystic nodule was the feature with highest likelihood ratio for benign nodules at 1:96 [74]. All children with suspicious thyroid nodules should also have a full US evaluation of their cervical lymph nodes. Thyroid nodules can also be detected incidentally on CT or as areas of focal uptake on 18FDG-PET scans performed for other reasons. When nodules are discovered incidentally, they should still be evaluated (with ultrasound) as differentiated thyroid cancer can be discovered in this way and increased uptake on 18FDG-PET is associated with an increased risk of thyroid cancer [75].
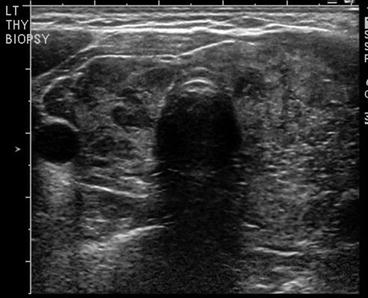

Fig. 14.3
Dominant nodule in the left lobe , heterogeneous appearance of the right thyroid lobe suggestive of Hashimoto’s thyroiditis
Lab Testing
Thyroid function tests should be checked in all patients with thyroid nodules, and in the absence of hyperthyroidism, an US-guided fine needle aspirate should be performed on any nodule with suspicious characteristics. Currently an elevated TSH level is not being used in practice to help predict the presence or absence of malignancy since results from studies conflict as to its clinical benefit [59–62].
Calcitonin levels are elevated in MTC and C-cell hyperplasia; measurement of calcitonin is not recommended in the absence of risk factors for MTC (positive family history of MTC or MEN2, presence of pheochromocytoma); this is mainly because the prevalence of sporadic MTC is very low in children. Thyroglobulin level is often elevated in thyroid disease, but this finding is not at all specific for thyroid cancer [76–78], and therefore, thyroglobulin testing should not be part of the diagnostic work-up of a thyroid nodule.
Thyroid Scintigraphy
TSH suppression may be found in a child with a nodule. In this situation, a radioisotope scan (iodine-123 or 99mTc pertechnetate) should be performed [79, 80]. A toxic adenoma will show increased nodule-specific uptake on the scan (a “hot nodule”). In adults, the presence of an autonomous or “hot” nodule is generally accepted as predictive of benign disease, and these patients do not require biopsy [13]. In children there are fewer studies of hot nodules, and they show some conflicting results: a study by Niedziela et al. in 2002 showed that in an area with iodine deficiency and recent use of iodine supplementation, there was an increased prevalence of hot nodules in children. Thirty-one children with hot nodules were followed, and 9/31 (29%) were diagnosed with differentiated thyroid cancer [79]. A separate study by Hodax found that in patients <21 years of age with thyroid nodules, 17/242 were autonomously functioning; six of these patients had surgery, and only one was diagnosed with papillary thyroid cancer [81]. In a recent study by Ly and colleagues, of 31 children with autonomous nodules (when the strict criteria of autonomy were applied), 21 had either a biopsy or surgical resection with histology, and 0/21 had thyroid cancer compared to 28/125 (22%) of their comparative group of children with non-autonomous nodules [82]. In the absence of long-term follow-up of alternative treatment modalities for children with hot nodules, the current ATA guidelines recommend that all symptomatic children with hyperthyroidism requiring treatment due to autonomous nodules proceed to surgical resection [71]; asymptomatic children or those with subclinical hyperthyroidism can be observed.
Fine Needle Aspiration Biopsy
The most recent ATA guidelines recommend that all FNA in children should be performed under ultrasound guidance [71]; this recommendation is based on the fact that thyroid nodules in children more commonly harbor malignancy than in adults [10] and that biopsies are more difficult to repeat in children. Cytopathology findings for children are classified in the same way as for adults: Bethesda system for reporting thyroid cytopathology [83, 84].
There are six categories, as follows:
- I
Nondiagnostic or unsatisfactory (the specimen has limited cellularity, absence of follicular cells, or poor preservation of cells)
- II
Benign
- III
Atypia or follicular lesion of unknown significance (AUS/FLUS)
- IV
Follicular/Hürthle cell neoplasm or suspicious for follicular/Hürthle cell neoplasm
- V
Suggestive of malignancy
- VI
Malignant
The sensitivity, specificity, and overall accuracy of FNA in children are considered similar to adults [85–90]. Table 14.2 shows the cytological outcomes of thyroid FNA biopsies reported in the pediatric literature over a 21-year timespan. The six Bethesda categories were not consistently used over this time period, and therefore for the purposes of comparison, Bethesda categories III and IV were grouped together into “atypical,” and at times III–V were grouped (this grouping is also called indeterminate). The prevalence of Bethesda I ranged from 4–28%, II 46–82%, III–V 3–38%, and VI 3–20%. The wide variation in results likely resulted from geographical differences between studies and variation in clinical assessment algorithms regarding when a biopsy should be performed. When all of the date are pooled (1551 nodules), the prevalence of Bethesda category I was 16%, 57% were Bethesda II, 18% were Bethesda III–V, and 10% were Bethesda VI.
Table 14.2
Cytological outcomes of fine needle aspiration biopsy of thyroid nodules in children
Author | Year published | Nodule number | Age, mean (range) | Inadequate (I) | Benign (II) | Atypical (III and IV) | Suspicious (V) | Malignant (VI) |
|---|---|---|---|---|---|---|---|---|
Raab [162] | 1995 | 66 | 13.1 (1–18) | 3 (5%) | 51 (77%) | 8 (12%) | 4 (6%) | |
Lugo-Vicente [163] | 1998 | 18 | 14.9 (9–18) | 3 (17%) | 11 (61%) | 2 (11%) | 2 (11%) | |
Khurana [164] | 1999 | 57 | 16.5 | 36 (63%) | 14 (25%) | 7 (12%) | ||
Al-Shaikh [165] | 2001 | 41 | 13.3 | 3 (7%) | 30 (73%) | 6 (15%) | 2 (5%) | |
Arda [166] | 2001 | 46 | 9 (5–11.6) | 2 (4%) | 33 (72%) | 5 (11%) | 3 (7%) | 3b (7%) |
Amrikachi [86] | 2005 | 218 | (10–21) | 62 (28%) | 119 (55%) | 20 (9%) | 17 (8%) | |
Hosler [167] | 2006 | 101 | 14.6 (8–18) | 13 (13%) | 48 (47%) | 13 (13%) | 5 (5%) | 22c (22%) |
Altincik [168] | 2010 | 30 | 4 (13%) | 24 (80%) | 1 (3%) | 1 (3%) | ||
Corrias [169] | 2010 | 104 | 11.5 | 7a (7%) | 77 (76%) | 8 (8%) | 19 (19%) | |
Monaco [17] | 2012 | 179 | 16.5 (4–20) | 21 (12%) | 82 (46%) | 62 (35%) | 6 (3%) | 8 (4%) |
Gupta [10] | 2013 | 136 | 13 (10%) | 86 (63%) | 16 (12%) | 10 (7%) | 11 (8%) | |
Norlen [170] | 2015 | 66 | 13.6 | 7 (11%) | 38 (58%) | 15 (23%) | 3 (4%) | 3 (4%) |
Lale [171] | 2015 | 282 | 59 (21%) | 136 (48%) | 46 (16%) | 6 (2%) | 35 (12%) | |
Amirazodi [172] | 2015 | 207 | (2–18) | 54 (26%) | 108 (52%) | 17 (8%) | 10 (5%) | 18 (9%) |
Pooled data | 1551 | 251 (16%) | 879 (57%) | 276 (18%) | 152 (10%) | |||
In adults, the risk of malignancy after an inadequate sample is 1–4% [83]; nodules with indeterminate cytology have a 5–30% risk of malignancy (lower in the AUS/FLUS lesions and higher in the follicular neoplasm or suggestive of neoplasm group) [84]. Published pediatric series are outlined in Table 14.3. The prevalence rates of malignancy using pooled data from these studies with a total of 561 histologic diagnoses showed a cancer prevalence of 4% (range 0–10%) after an inadequate(Bethesda I) FNA sample, 8% (range 0–33%) after benign(Bethesda II) cytology, 48% after an indeterminate (Bethesda III–V) FNA sample, and 100% after malignant (Bethesda VI) FNA. Since most nodules with benign cytology on FNA are managed nonoperatively, these retrospective studies of operative cases significantly overestimate the prevalence of malignancy in the benign (Bethesda II) category: cancer prevalence rates vary from 0 to 33% in various studies (pooled data shows a prevalence of 8%). The children reported in these studies were taken to surgery despite negative FNA, presumably because of some concerning features on physical exam or ultrasound; therefore, higher risk nodules are overrepresented in the surgical group. Assuming all nonoperative benign (Bethesda category II) FNA samples are actually benign, the prevalence rate for malignancy in the pooled data drops from 8 to 2%; the true frequency of malignancy in this group likely falls somewhere between these two rates.
Table 14.3
Histologic correlation of cytology results in pediatric patients, reported as number of malignancies found for each category
Author | Number of nodules with histology | Inadequate (I) | Benign (II) Assuming all nonoperative were benign | Atypia/FLUS (III) | FN/SFN (IV) | Suspicious | Malignant |
|---|---|---|---|---|---|---|---|
Raab [162] | 25 | 1/13 (8%) 1/51 (2%) | 4/8 (50%) | 4/4 (100%) | |||
Lugo-Vicente [163] | 15 | 2/11 (18%) 2/11 (18%) | 1/2 (50%) | 2/2 (100%) | |||
Gupta [10] | 63 | 1/13 (8%) | 2/14 (14%) 2/63 (3%) | 4/9 (44%) | 6/6 (100%) | 4/10 (40%) | 11/11 (100%) |
Corrias [169] | 55 | 0/30 (0%) 0/77 (0%) | 0/6 (0%) | 19/19 (100%) | |||
Khurana [164] | 24 | 1/3 (33%) 1/36 (3%) | 6/14 (43%) | 7/7 (100%) | |||
Altincik [168] | 5 | 1/4 (25%) 1/24 (4%) | 1/1 (100%) | ||||
Arda [166] | 31 | 0/2 (0%) | 0/18 (0%) 0/33 (0%) | 0/5 (0%) | 1/3 (33%) | 3/3a (100%) | |
Amrikachi [86] | 32 | 0/1 (0%) | 0/11 (0%) 0/119 (0%) | 4/9 (44%) | 11/11 (100%) | ||
Hosler [167] | 45 | 0/1 (0%) | 4/15 (27%) 4/48 (8%) | 3/8 (38%) | 2/5 (40%) | 13/16 (100%) | |
Monaco [17] | 96 | 0/8 (0%) | 2/30 (7%) 2/82 (2%) | 7/25 (28%) | 11/19 (58%) | 6/6 (100%) | 8/8 (100%) |
Norlen [170] | 31 | 0/3 (0%) | 0/9 (0%) 0/38 (0%) | 2/9 (22%) | 4/4 (100%) | 3/3 (100%) | 3/3 (100%) |
Lale [171] | 74 | 1/10 (10%) | 0/17 (0%) 0/136 (0%) | 2/4 (50%) | 7/18 (39%) | 4/4 (100%) | 24/24 (100%) |
Amirazodi [172] | 65 | 0/12 (0%) | 3/19 (16%) 3/108 (3%) | 6/9 (67%) | 5/7 (71%) | 18/18 (100%) | |
Pooled | 561 | 2/50 (4%) | 16/194 (8%) 16/826 (2%) | 92/193 (48%) | 124/124 (100%) | ||
In view of these findings, children with suspicious nodules on ultrasound need follow-up even if the FNA is inadequate or benign. When cytopathology results are inadequate, a repeat US and FNA should be performed 3–6 months later; it should not be performed sooner due to the risk that the FNA can change US features of the nodule [91] and result in atypical cellular features during the healing process [92].
Molecular Genetic Testing
Another modality used in adults to help differentiate between benign and malignant disease is molecular genetic testing [93–98]. This topic is reviewed extensively elsewhere in this book. Multiple studies show that the mutations found in pediatric thyroid cancer differ from those of adults [99] and that the genetics of radiation-exposed and sporadic thyroid cancers are very different [99–101]. Genetic rearrangements including RET/PTC are far more common in children than in adults, especially in children with radiation-exposed thyroid cancers: studies show a pooled prevalence of RET/PTC rearrangements in radiation-exposed pediatric PTC of 58% (range 33–76%) [101–109] and 41% in sporadic pediatric PTC (range 15–65%) [99–101, 107, 108, 110–122], compared to 10–20% of adults [123–125].
BRAF mutations are the most common mutations in adult PTC, seen in 61.7% (range 29–83%) of patients [126–129]; they are less common in children where studies on sporadic papillary cancer show a pooled prevalence of BRAFV600E of 13% [99], albeit with great variation between studies ranging from 0 to 63% [99–101, 107, 108, 110–122]. In radiation-exposed PTC, the prevalence of BRAF mutations in the few studies performed is very low at 3%, with a range of 0–8% [103–111] and no BRAF mutations have been found in a child less than 10 years of age [99].
The goal of using mutational analysis on cytology samples is to help differentiate between benign and malignant disease. Using the Bethesda cytology classification system, pediatric studies show that category VI is highly predictive of malignancy (100% in the pooled studies presented in Table 14.3), while the rate of malignancy was still 48% in the indeterminate categories (III, IV, and V), suggesting that molecular studies in this group could prove useful. Pediatric studies have shown that the presence of a mutation on cytology is highly predictive of malignancy [17, 130]; however, in the largest study to date, mutation-negative FNA samples still had a 48.5% malignancy rate [17]; therefore, molecular analysis of cytology samples is not currently recommended for children [71].
Since thyroid nodules in children are more commonly malignant, follow-up of even benign lesions is important. Follow-up should be by US 6–12 months after the initial benign FNA.
Management of Thyroid Nodules
Management of thyroid nodules depends on the diagnostic work-up and consists of either surgery or potentially levothyroxine therapy. Nodules which are predominantly cystic and have no concerning US features require neither FNA nor treatment.
Surgical Management of Thyroid Nodules
The risks and benefits of surgery for thyroid nodules are based on the risk of thyroid cancer predominantly assessed by the Bethesda category on FNA [71]. The higher the risk of malignancy, the more likely surgery is necessary and the more extensive that surgery should be. Thyroid surgery in children should be carried out by high-volume thyroid surgeons to decrease the potential for complications [131–133]. All children undergoing thyroid surgery should have a full neck ultrasound to determine potential extent of disease and therefore the extent of surgery. Children with papillary thyroid carcinoma (by far the most common thyroid cancer in children) should undergo a total or near-total thyroidectomy due to an increased prevalence of bilateral (30%) and multifocal (65%) disease in children [134–136]. Studies have shown a lower risk of recurrence when a total or near-total thyroidectomy is performed [135–137]. Bilateral lobar resection compared very favorably to lobar resection in a study of 215 children and adolescents followed for 40 years: it improved recurrence risk from 35% to 6% [137]. Children with thyroid cancer may also require central and potentially lateral neck dissections depending on the presence of extra-thyroidal invasion or metastatic lymph nodes [71].
Surgery may be indicated in the absence of confirmed differentiated thyroid cancer; the most recent ATA guidelines recommend surgery (at least a lobectomy and isthmectomy) for any indeterminate cytology on FNA (Bethesda III–V) due to the high risk of cancer in these patients (48% in the pooled data in Table 14.3). We advocate the use of intraoperative frozen section histology to determine the need or not for completion thyroidectomy; this is very beneficial in cases of classic papillary thyroid carcinoma, but it is less helpful for follicular variant PTC and is not helpful for follicular thyroid carcinoma since their diagnosis depend on histologic evaluation of the entire lesion for evidence of vascular or capsular invasion [138–140].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree