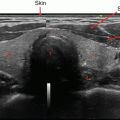
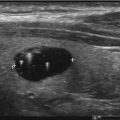
Fig. 13.1
Percutaneous ethanol injection. (a) US scan of the left thyroid lobe. The presence of a large thyroid cyst, relapsing after a former percutaneous drainage. Benign cytology. (b) Thyroid US scan performed 1 h after the drainage of the fluid collection followed by US-guided injection of ethanol into the cavity. (c) The same lesion 6 months after treatment. Complete disappearance of the fluid collection with marked shrinkage of the cystic thyroid nodule. (d) Contrast-enhanced US performed 6 h after the procedure. A large area of tissue within the nodule is now ablated, as demonstrated by the absence of blood supply after the intravenous enjection of contrast medium
Indications for Clinical Practice
The rare pure cysts, and the more common frequent thyroid nodules with a major fluid component, are treated effectively with PEI [12]. PEI should be performed in cysts and complex nodules that relapse after drainage and gradually increase in size or are symptomatic. Before treatment a repeat cytological evaluation of both the fluid and the solid component of the lesion should be performed to rule out the risk of a cystic carcinoma. The procedure is rapid, well tolerated, and safe, if appropriately performed, and recurrent cystic lesions result in a clinically significant reduction of the size (usually greater than 80% in randomized trials) [20, 21] and improvement of local symptoms [19, 22]. Volume decrease is long-lasting, as demonstrated by the absence of volume changes during long-term follow-up [22, 23].
Complications and Limitations
The occurrence of vocal cord paresis is rare and transient and is always due to the incorrect injection of ethanol outside the cystic cavity. No loss of thyroid function nor autoimmune disease occurs after PEI. Of note, in case of large cystic lesions, two or more treatments are generally needed during a 1-month period. The occasional occurrence of a rapid hemorrhagic refilling of the cyst during fluid drainage is associated with a less favorable outcome [12].
Solid thyroid nodules, either cold or hyperfunctioning, are less suitable for PEI [3] even if the procedure may result in a nearly 50% volume decrease [23, 24]. In these lesions ethanol injection is painful, multiple treatments are required, and relevant side effects may follow. Due to the unpredictable distribution of ethanol within solid tissue and its leakage into the thyroid gland and the neck tissues, patients usually complain of a nasty cervical pain that radiates to the jaw and thorax [11]. Complications are much more frequent than in cystic lesions, and the procedure may result in a fibrosis of the neck structures that, if requested, may turn into a difficult surgical approach [12].
Laser Ablation
Procedure
Before laser ablation a careful administration of local anesthesia, xylocaine, on the skin, prethyroid muscles, and thyroid capsule should be performed along the planned track of fiber insertion. Usually, from one to two 21G spinal needles are inserted under US guidance into the nodule. A 300 μm optical fiber is then positioned through the needle sheath into the target zone, and the tip is placed in direct contact with the tissue for a length of about 5 mm [25, 26]. A careful US evaluation should be performed to confirm the appropriate positioning of the fiber optic(s) and the presence of a 10 mm safety distance from the vital structures of the neck. Then, laser firing is initiated with an output power from 3 to 5 W, and the energy delivery is usually carried out for 5–10 min, with a total energy delivery from 1800 to 3000 J. Laser illumination is terminated when a cloud of hyperechoic spots, due to the production of gas bubbles, stops enlarging and becomes steady in size. In large volume nodules, a second treatment may be carried out during the same session, after a 1.0–1.5 cm withdrawal of the needle(s) along the longitudinal axis of the lesion (“pullback technique”) [27] (Fig. 13.2).
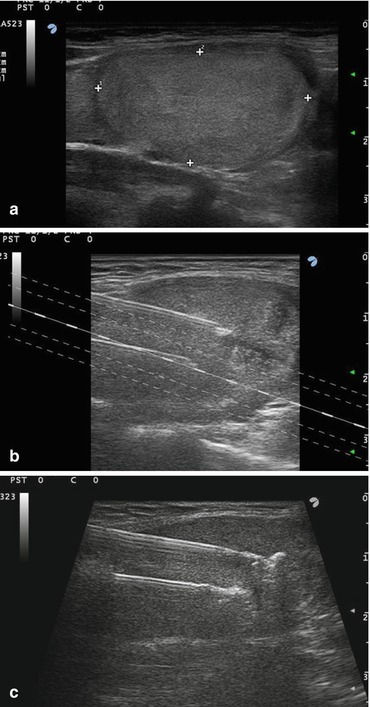

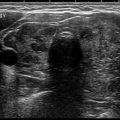
Fig. 13.2
(a–c) Percutaneous laser ablation. (a) US scan of the right thyroid lobe. Presence of an isoechoic nodule with regular margins, 30 mm in its greater diameter, progressively enlarging over the years. A former fine needle biopsy resulted in a benign cytologic sample. (b) US-guided insertion of two needles within the lower part of the nodule. (c) US detection of the initial hyperechoic spots due to laser firing
Laser treatment is rapid and is usually fairly well tolerated. A mild cervical pain, sometimes radiating to the chest and described by a minority of patients, resolves within an hour and is well responsive to common analgesics. Low-grade hyperthermia is rarely observed after the procedure. No cosmetic damage is induced by the procedure, and cervical bleeding, skin bruising or swelling, and abnormalities of thyroid function are definitely uncommon. The risk of major complications (vocal cord paresis or injury to cervical structures) is well under 1%, is always caused by an incorrect procedure, and was mostly reported during the learning curve of the technique [12, 28].
Indications for Clinical Practice
Laser ablation is successful for decreasing the size of large symptomatic thyroid lesions and for preventing further growth of nonfunctioning nodules. A good-quality evidence, due to randomized trials [29, 30] and multicenter retrospective studies [28], demonstrates that a single LA session is consistently followed by about 50% reduction of the size of the ablated nodule. Volume decrease is progressive during the first 12 months after treatment and in most cases persists for several years. These results are quite similar among different centers and are associated with a significant improvement of local symptoms [28].
Laser ablation is employed less frequently nowadays for hyperfunctioning nodules because radioiodine treatment is readily available, safe, and a highly effective alternative to surgery for the control of hyperthyroidism and, to a lesser extent, nodule growth. LA should be considered for patients with small-size autonomously functioning nodules that incompletely suppress normal thyroid tissue and are at risk of hypothyroidism after radioiodine therapy [31]. LA is strongly indicated in subjects for which radiation exposure is inappropriate, due to pediatric age or pregnancy, or radioiodine cannot be used because of an iodine-replete condition caused by drugs or contrast media. Finally, in patients with large toxic nodules that are at surgical risk, the combined management with initial LA followed by radioiodine treatment results in a rapid control of local pressure symptoms [32, 33].
LA may be used for complex lesions with a large solid part to induce with a single-session liquid evacuation and shrinkage of the solid component, but PEI remains the first-line, nonsurgical treatment for relapsing cystic nodules [34].
Radiofrequency Ablation
Radiofrequency devices generate an alternating electric field within the target lesion that is produced by an electrode needle connected to an external radiofrequency generator. The fast movements of the ions are followed by progressive heating of the tissue and, eventually, by thermal necrosis [35].
Procedure
Radiofrequency ablation may be performed with local anesthesia or conscious sedation. After the initial trials are carried out in subjects under conscious sedation with large devices, such as multi-tined electrodes with expanding hooks (14-gauge diameter) [36, 37], thinner (18 or 19G) and shorter, internally cooled electrode needles have been developed and are presently used for thyroid lesions [38]. These tools are more practical and less traumatic and permit a repetitive insertion of the tip of the needle within the target area, according to the so-called “moving shot” technique [38]. The electrode is first placed, through a cervical trans-isthmic approach, in the distal part of the nodule, and after the ablation of the initial zone, it is systematically inserted into the central and more proximal areas of the thyroid lesion (Fig. 13.3).
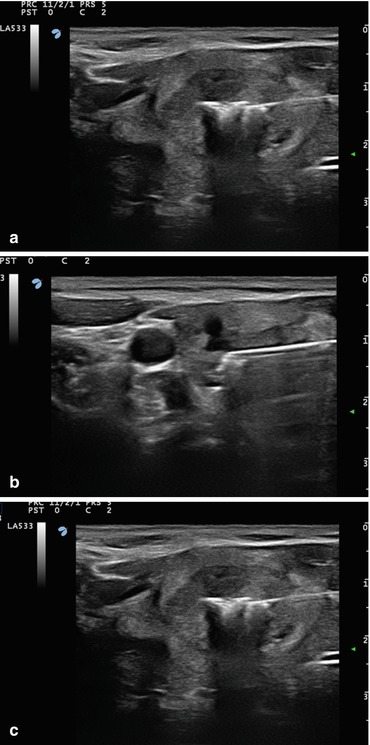
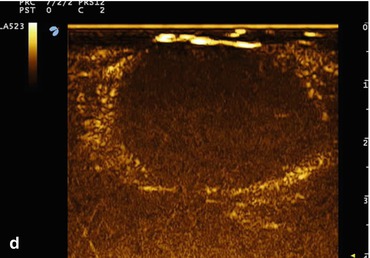


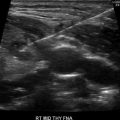
Fig. 13.3
Percutaneous radiofrequency ablation . (a–c) US monitoring of a treatment with an 18G radiofrequency electrode needle of a symptomatic thyroid nodule, benign at fine needle aspiration biopsy. The tip of the electrode needle is sequentially moved within the nodule producing multiple areas of thermal necrosis. (d) Contrast-enhanced US performed 6 h after the procedure. A large area of tissue within the nodule is now ablated as demonstrated by the absence of blood supply
Indications for Clinical Practice
The “moving shot” RF technique [39, 40] generates multiple confluent areas of thermal necrosis that are followed by a relevant shrinkage of the ablated lesion. Several RF trials report a mean size reduction that 6 months after treatment is about 60–80% and may reach 90% of the baseline volume in complex lesions with a fluid component [40]. Volume reduction is persistent over time, and further treatments may be, anyway, performed in case of nodule regrowth [40]. RF ablation is moderately painful, can be carried out in the outpatient clinic, and does not require major analgesics nor antibiotics. Side effects are rather infrequent (about 3.0% in large retrospective studies) and are usually transient [41]. Rare but serious complications, such as extra-thyroid bleeding, vocal cord palsy, nodule rupture and infection, and skin injury, have been described. Thus, RF ablation requires special and specific expertise with a good knowledge of cervical anatomy that should be obtained with a dedicated training period [41].
Similar to laser ablation, RF may be used for the ablation of hyperfunctioning thyroid nodules [42, 43]. Despite noncontrolled and controlled trials reporting successful results in toxic thyroid nodules [42, 43], RF, like laser ablation, rarely results incomplete destruction of the peripheral areas of the hyperfunctioning nodule. Due to the potential persistence of autonomously functioning tissue and the risk of hyperthyroidism recurrence, RF may, seldom and selectively, be used in patients with contraindications to radioiodine treatment.
RF ablation is rarely used for the treatment of cysts or thyroid nodules that are prevalently fluid. RF may induce with a single procedure a shrinkage of large cystic lesions that is similar to that achieved with multiple PEI sessions [44]. However, due to the efficacy, easiness, and safety of PEI treatment, percutaneous ethanol injection remains the first-line procedure for the management of relapsing thyroid cysts [45].
HIFU
During HIFU treatment several externally generated US beams are focused on a target lesion within the body. Each individual beam passes through the skin with minimal damage, while at the focal point, the union of the multiple beams of ultrasound waves results in tissue heating, denaturation of cell proteins, and coagulative necrosis. Furthermore, focused ultrasound can disrupt the cells through a mechanical action, due to the oscillation of gas bubbles in the US field, that is independent from tissue heating [46].
Procedure
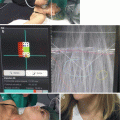
The treatment is performed with a real-time US-guided HIFU system that consists of an energy generator, an articulated arm with a treatment head, a cooling system for skin preservation, and a touch screen interface for planning and follow-up of the procedure. The treatment head includes both the imaging transducer and the HIFU transducer that convey energy to the target area. A laser-based movement detector shuts off the power in case of patient movement or swallowing.
Patients are placed in supine position with hyperextended neck and given a mild sedation. After the definition of the treatment volume and of the boundaries of vital structures (carotid artery, trachea, and skin) on a touch screen interface on two axes, HIFU pulses are sequentially delivered to the target lesion. During the procedure the operator controls the beam focus and, if necessary, repositions the treatment head. The applied energy is adjusted according either to the evidence of the hyperechoic marks due to tissue coagulation or to the occurrence of cervical pain [47] (Fig. 13.4).
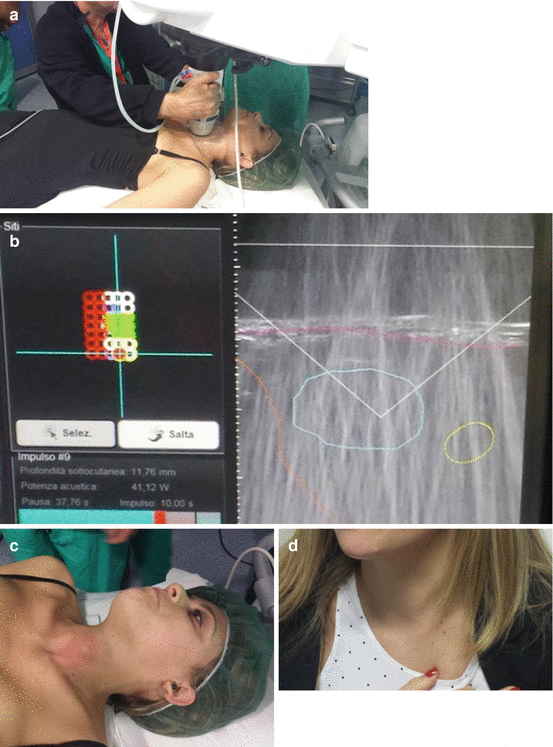

Fig. 13.4
HIFU ablation . (a) Positioning of the HIFU robotic arm equipped with the US transducer over the neck of the patient. (b) Left. Targeting for HIFU treatment of the safe areas (green) and of the areas at risk (red) of thyroid nodule. Right. Monitoring of the shot of high-intensity US waves focused into the central zone of the thyroid nodule. (c) At the end of HIFU treatment, a hyperemic area is visible on the skin of the neck, mainly due to the pressure of the cooling device of the US probe. (d) Nearly complete disappearance of the skin redness 4 h after treatment
Indications for Clinical Practice
In few small series of patients, the treated nodules showed at the 6-month follow-up a volume reduction of nearly 50% [47, 48]. The procedure was reported as fairly well tolerated, and most patients did not need analgesic drugs after treatment. At the session conclusion, mild skin redness and subcutaneous edema spontaneously disappearing within a few hours or days may be observed. Blistering or severe edema of the cervical tissues is occasionally reported, while serious adverse events, such as dysphonia, tracheal or esophageal damage, or Horner’s syndrome, are rare [47].
The use of HIFU has still some limitations in clinical practice. The available evidence is of low quality because it is based on small-size uncontrolled trials, with short follow-up and performed in a limited number of centers. The area to be treated and the beam track should be placed at safety distance from the skin and vital cervical structures, and thus part of the nodules is treated with difficulty with this technique. Finally, when compared to the other MIT procedures, the equipment is more expensive and less accessible, and the treatment time is more protracted. Larger prospective randomized trials, with strict enrollment criteria and appropriate follow-up, are needed for the definition of the role of this potentially innovative procedure.
Microwave Ablation (MW)
Microwave ablation is a minimally invasive technique that has been used to treat benign and malignant tumors of the liver, lung, and kidney [48]. Presently, only few studies report the experience on benign or malignant thyroid nodules. A large retrospective study was carried out in China on 254 benign thyroid nodules that were treated with the use of a 16G microwave antenna [49] under local anesthesia. At a 6-month follow-up, the mean decrease in the volume of thyroid nodules was greater than 50% versus baseline. The treatment was reported as well tolerated, but pain and ill-defined voice changes were registered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree