Introduction
Normal pressure hydrocephalus (NPH) is a condition that is characterized by the clinical triad of gait disturbance, cognitive decline and urinary incontinence. It is further supported by imaging evidence of ventriculomegaly. The typical age group involved is above 60 years, although it can occur as early as 50 years,. Because it generally occurs in the geriatric population, detection may be challenging as the symptoms overlap with those of other degenerative neurological conditions and sometimes the decline may even be attributed simply to old age. It is therefore important that NPH be kept in mind when evaluating relevant patients. Its incidence has been estimated at 1.8 cases per 100 000,1 but others have it higher at 5.5 per 100 000.2 One study even estimated that the prevalence may be 14% in assisted-living facilities.3 Regardless of the numbers, many feel that it is an under-recognized condition so there has been a push to highlight its importance not just among healthcare providers but also the general population so that family members may bring potential patients to medical attention.
NPH is widely accepted to have been first identified in 1965,4 but it was recognized as early as 1957 by Dr. Salomon Hakim, who is regarded as the father of NPH.5 The name was derived from his observation that such patients did not present with papilloedema and had normal cerebrospinal fluid (CSF) pressures on lumbar puncture. It was a revolutionary discovery as it gave new hope that a degenerative neurological condition could now be reversed through simple CSF diversion. It should be noted that although the term ‘normal pressure hydrocephalus’ is widely used, changing concepts have given rise to questions on the validity of this nomenclature. Some authors have suggested, for example, that CSF pressure in NPH is not exactly ‘normal’ as some show abnormalities in CSF dynamics.6 Terms such as ‘chronic hydrocephalus’ have been proposed, but thus far none have been convincing enough to cause a shift away from the widely used term ‘NPH’.7
Aetiology and Pathophysiology
Hydrocephalus by definition is an excessive accumulation of CSF in the ventricular system. What leads to this condition can be a host of causes ranging from haemorrhage to neoplasm. However, its aetiology can be roughly divided into two categories: non-communicating, wherein there is a blockage in the ventricular system (e.g. aqueductal stenosis); and communicating, which can result either from a decrease in CSF resorption (scarring after subarachnoid haemorrhage) or from CSF overproduction (e.g. choroid plexus papilloma). In the realm of geriatrics, it is virtually never a consequence of CSF overproduction. As far as NPH is concerned, it is commonly accepted that fluid build-up is due to impairment of the brain’s CSF resorptive capacity—an idea that is known as the bulk flow theory. This can occur at the level of the arachnoid granulations along the skull base and over the hemispheres.8 Some authors feel that resorption also occurs in the brain parenchyma through capillaries and venules9, 10 and resorptive problems can also be at this level. Among the known causes of NPH are subarachnoid haemorrhage, trauma and meningitis, all of which can lead to scarring of the arachnoid granulations in the subarachnoid space. Such conditions are commonly referred to as secondary NPH.
In over half of NPH patients, however, there is no identifiable cause. The development of idiopathic NPH remains unclear, but it appears to involve the interplay of several factors such as cerebral ischaemia, decreased vascular compliance, decreased cerebral blood flow to periventricular regions and brain atrophy. MR flow quantification studies showed lower venous compliance in NPH patients, which could lead to increased resistance to CSF outflow and cerebral ischaemia in the concerned areas.11 Indeed, it has been shown that after shunting, cortical vein compliance is improved in patients who respond to the procedure.12 The bulk flow theory is easily understandable but others feel that it is an oversimplification. It appears inadequate in explaining certain characteristics such as why CSF pressure can remain normal and why some patients improve with endoscopic third ventriculostomy (ETV), which does not involve diverting fluid volume away from the intracranial space. An alternative theory known as the hydrodynamic concept of hydrocephalus has been gaining traction. It holds that a decrease in intracranial compliance allows an increased transmission of systolic pressure to the brain parenchyma, which in turn becomes distended against the unforgiving skull.13 Creating a hole on the floor of the third ventricle (ETV) thus allows for ‘venting’ of the increased systolic pressure and CSF to the subarachnoid space. The thought is that the aqueduct of Sylvius is too narrow and inadequate for venting.
There are other factors that influence the pathophysiology of NPH. Cerebral blood flow has been shown to be decreased in patients with idiopathic NPH, particularly in periventricular regions14 and also the basal ganglia and thalamus.15 Idiopathic NPH patients have also been found to have brain atrophy in addition to hydrocephalic features.16 More recently, there has been an increasing awareness of the role that metabolic disturbances play in the genesis of NPH symptoms. For instance, accumulation of beta-amyloid and disturbance in cholinergic neurotransmission may contribute to cognitive and memory decline.17 This would explain why cognitive function sometimes does not improve after shunting. It is also suggested that deterioration of striatal GABAergic neurons and lower dopamine levels in the substantia nigra could lead to motor dysfunction. This idea would account for the observation that patients who initially show gait improvement after shunting later regress despite proof that the shunt is working or even after valve pressure adjustments. Although we do not fully understand the interplay of the multiple factors that affect NPH patients, it is clear that NPH symptoms do not simply evolve from problems in CSF dynamics.
Clinical Presentation
Although NPH has traditionally been associated with neurosurgeons because of its ultimate treatment, the evaluation and workup that lead to surgery require the involvement of many other medical professionals. It may be the primary care provider who casts the first suspicion or the geriatric specialist who pushes for further workup. Then there is the neuropsychologist who delineates the condition by performing neurocognitive evaluations. However, it can be argued that it is the neurologist who holds the central position as he or she has the deepest knowledge of other similar conditions that allow for better differentiation and diagnosis of NPH.18
It is easy to say that NPH presents with the classic triad of gait disturbance, urinary incontinence and mental decline. However, it can be challenging to assess the character of each of these symptoms since there are other conditions that may produce NPH’s components. For instance, gait disturbance can be seen in movement disorders and a common differential is Parkinson’s disease. Memory or cognitive decline can be seen in Alzheimer’s disease; in fact, it is the more common consideration. As for bladder incontinence, there are a host of urological and neurological reasons that can be suspected. Having said all that, there are typical characteristics that we can look for when evaluating a patient who presents with these symptoms.
The most prominent symptom is usually the progressive difficulty in ambulation. It is typically described as a wide-based gait that is slow and unsteady. The patient usually walks in small steps and has poor floor clearance, that is, they do this with their feet clearing the floor at a low height. Others describe it as a feeling that the feet are glued to the floor, commonly referred to as ‘magnetic feet’. The degree of gait disturbance depends on the severity of the NPH and patients who are in the early stages of the disease can walk almost normally except for some unsteadiness. It is not uncommon to encounter patients who are still highly functional and simply report a gait disturbance on walking a certain distance or against an incline. Conversely, patients with severe NPH can be so debilitated that they are unable to stand without support. Despite the advanced and severe state, their response to shunting can be very dramatic so they are not to be dismissed or given up on.
Mental decline is usually in the form of problems with memory, particularly short-term memory. It may also involve a decrease in mental alertness and an overall slowing of thought processes. The process may be so gradual that nobody notices it or it is attributed to ageing. It is important to ask family members aside from the patient because it may not be apparent to the patient himself or herself. The physician may also find it difficult to detect during the finite visit, whereas family members are able to share their observations of the patient over a longer period of time and across varying situations. Family members are crucial to the history-taking in this sense.
Urinary incontinence may start as progressive urgency, but many will have frank incontinence by the time of consultation. It is a symptom that the examiner needs specifically to ask about because it may not be recognized by the elderly patient or family members as significant. They may not realize that it is related to the overall neurological condition or the patient may be somewhat embarrassed to say that he or she is already on adult diapers. Of the clinical triad of symptoms, urinary incontinence is usually the last to develop. It is considered to be a symptom of late-stage NPH.19
Differential Diagnosis
When dealing with the geriatric population, it is not hard to imagine that NPH symptomatology in an elderly patient can be easily mistaken for, and brushed aside as, part of the ageing process. Awareness of this condition is not as prevalent as it should be and many patients do not come to medical attention. Indeed, one can only wonder how many patients have gone undiagnosed and have potentially missed out on a better quality of life had they been shunted.
Another challenge to identifying NPH is related to its differential diagnoses. These include Parkinson’s disease, Alzheimer’s disease, vascular dementia and spinal stenosis. Most commonly, it can be difficult to differentiate from Parkinson’s and Alzheimer’s disease. Gait similarities in NPH and Parkinson’s disease include short steps and leg rigidity.20 The typical tremor of Parkinson’s disease may help make this diagnosis, but sometimes patients with NPH can also have confusing tremors. In general, it is possible to differentiate between the two by the gait patterns. NPH is characterized by reduced stride length, reduced step height and balance difficulty. Parkinson’s disease patients have a shuffling gait with impaired arm swing and walk with their hips and knees slightly flexed and trunk bent forward.21 In comparing Alzheimer’s disease and NPH, the dementia is not as pronounced as in Alzheimer’s disease. However, it is not uncommon to find NPH patients with concurrent Alzheimer’s disease.22 It is important to identify patients with both Alzheimer’s disease and NPH, because this particular subset tends to respond poorly to shunting.23 Given these challenges in differential diagnosis, we again emphasize the crucial role that neurologists and neuropsychologists play in the evaluation of potential NPH patients.
Diagnostic Modalities
Numerous tests have been developed over the years to help in the diagnosis of NPH. Some have stuck and become incorporated into regular practice whereas others have passed on to the realm of historical interest. It is fascinating to see how the evolution of these tests reflects the dynamic ideas that have been elucidated with regard to its pathophysiology over the past five decades. As in many medical conditions, diagnostic tests are not meant to replace good history taking and physical examination. In fact, clinical acumen and computed tomography/magnetic resonance imaging (CT/MRI) play a primary role in clinching the diagnosis. However, it is important to note that the diagnostic workup for NPH is as much about diagnosis as it is about determining whether a patient will respond well to surgery. For this reason, a number of other diagnostic modalities have been developed to predict shunt responsiveness. There is still significant variability in practice as far as usage is concerned, depending on ideological and logistical differences from place to place.
CT/MRI
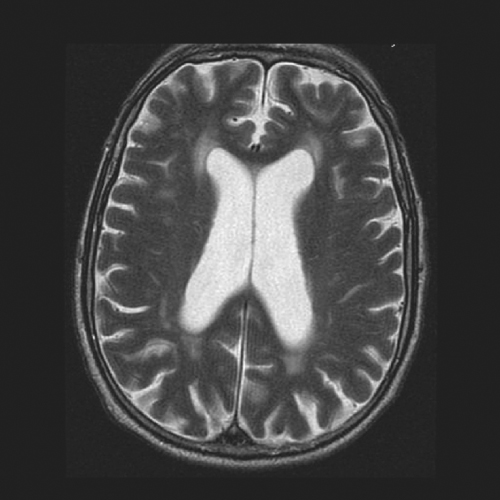
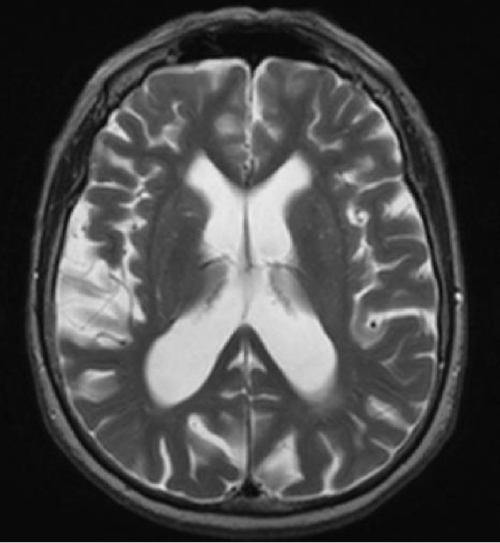
Imaging via CT or MRI remains the main diagnostic tool in NPH. The finding that is demonstrated is ventriculomegaly (Figure 56.1). Whereas a head CT is able to show enlarged ventricles, MRI gives additional information that may contribute to the evaluation of a patient. For instance, white matter changes in the subcortical and periventricular areas have been associated with NPH. These abnormalities appear on MRI as T2 hyperintensities (Figure 56.2). An MRI can also show flattened gyri and effaced sulci that indicate tightness in the intracranial compartment. It is important to remember, however,, that even if the arachnoid space looks ‘spacious’, it does not necessarily rule out the condition. MRI may also provide details that may be helpful in excluding other disease entities. For these reasons, it is probably better to order an MRI whenever feasible and it is unnecessary first to go through a CT scan. However, in places where MRI is not readily available, CT scanning is adequate for the purpose of demonstrating ventriculomegaly. It is also easier and cheaper to use CT in the postoperative period when checking for shunt position or monitoring for formation of subdural collections after shunt valve reprogramming (see below).
Figure 56.1 CT scan showing typical features of NPH, with ventricular enlargement out of proportion to cerebral atrophy.
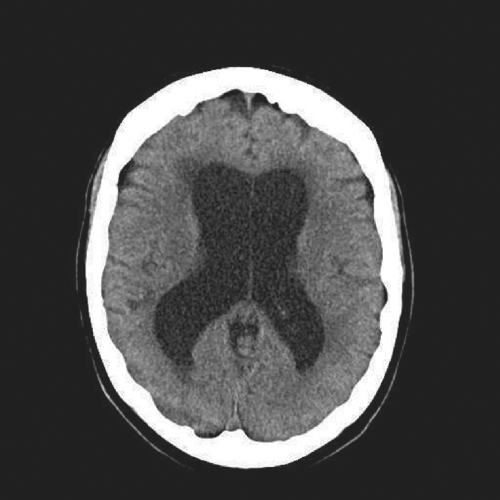
In the elderly population, it is common to find cerebral atrophy. This age-related thinning of the cerebral mantle is accompanied by a reciprocal dilatation of the CSF spaces including the ventricles, a finding that is commonly referred to as ‘ex vacuo dilatation’. Whether it is a CT or an MRI of the brain, this ex vacuo phenomenon has to be taken into consideration when evaluating for hydrocephalus in the geriatric population. For it to be significant, the ventriculomegaly has to be out of proportion to cerebral atrophy (Figure 56.3).
Figure 56.3 Natural ex vacuo dilatation of the ventricles as a result of ageing-related cerebral atrophy.
Lumbar Puncture
In many disease conditions, a lumbar puncture (LP) or spinal tap is routinely used to obtain CSF for laboratory studies. Its role is different in NPH; it is not so much for CSF sampling but rather CSF evacuation to see whether removal of CSF from a patient’s system results in an improvement of symptoms, primarily gait. The other difference is that larger amounts of CSF evacuation are necessary in the evaluation of NPH as smaller volumes may not be sufficient to uncover potential benefits. It is therefore typically referred to as a high-volume LP to differentiate it from the usual LP where just enough is obtained for cell counts or culture. The amount of CSF removed varies but is usually around 40 ml. Although any competent physician may choose to perform the LP, it is commonly done by the neurologist, and for good reason. Aside from being technically skilled in the procedure itself, neurologists are in the best position to evaluate the character of a patient’s gait before and after high-volume LP. For a more accurate comparison, many will videotape the patient walking pre- and post-LP. In patients who show response, there is usually a return to the pre-LP gait disturbance after a few weeks. This further solidifies the diagnosis.
Although CSF may be sent to the laboratory for other tests, it serves no purpose in the diagnosis of NPH. There are ongoing efforts to identify CSF biomarkers that would be helpful in the diagnosis of NPH, but none has been proven to be useful.24 Measuring CSF pressure for NPH per se is also unnecessary since the value of LP is in boosting the diagnosis of NPH and indicating shunt responsiveness if there is subsequent improvement in gait. Several studies have highlighted the high predictive value of a positive lumbar puncture test.25–27 There are doubts, however, about how well this test identifies candidates for ventriculoperitoneal (VP) shunting, because the amount of CSF removed in this one-time process can be inadequate in producing neurological improvement. One can argue that LP does not closely mimic the continuous drainage of shunting. Some worry that there could be patients who show no gait improvement after this momentary drainage of CSF and potentially lose out on benefiting from a shunt. Hence many still consider or at least discuss the option of shunt placement even if high-volume LP is equivocal or unrevealing, especially if clinical signs and symptoms are typical for NPH. Others would advocate a more detailed and involved test such as a lumbar drain trial (see below). Still, LP continues to be the mainstay in identifying appropriate shunt candidates. It is available anywhere and is logistically simple to administer.
Lumbar Drain Trial
To mimic better the potential effects of a shunt, many have moved on to performing a lumbar drain (LD) trial rather than just relying on an LP. LD involves performing a lumbar puncture using a large-bore Touhy needle followed by insertion of a small-calibre catheter. The catheter is left in place to drain CSF continuously from the thecal sac for ∼4 days. The catheter is connected to an external bag within a closed system that on average drains about 100–150 ml of CSF per day. Unlike LP, this is an in-house procedure and is usually under the care of the neurosurgery service. The patient stays in the hospital for the duration of the LD trial and is checked daily for improvement in gait and cognitive function. Typically, a third-party person such as a physiatrist or neurologist is in charge of the daily assessment so as to minimize bias. The choice between doing an LP or an LD trial varies across institutions not only due to differences in physician beliefs but also for logistical reasons such as bed availability for this type of non-urgent admission. Some patients also do not tolerate LD well. It may need to be aborted if there is significant spinal or low-pressure headache or nerve root irritation by the catheter causing back or leg pain.
LD has been shown to predict the surgical outcome in up to 100% of cases.28, 29 However, most would give an estimate that is closer to 80%. A prospective multicentre study found that a positive lumbar drain test predicted good shunt response in 87% of cases, but cautioned that a negative result in the test is unreliable as the false-negative rate was 64%.27 Therefore, patients who have a negative lumbar drain test should still be considered for shunting and one should look at the overall clinical picture and the patient’s wishes as guides in the decision-making process.
Lumbar or CSF Infusion Test
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree