Nonisotopic Techniques of Thyroid Imaging
Laszlo Hegedüs
Finn Noe Bennedbaek
Imaging has revolutionized the evaluation of patients with thyroid disease during the past three decades. However, it is important to bear in mind that thyroid imaging, useful as it may be, is in general not evidence-based, and there have been few cost–benefit evaluations of these procedures (1).
The thyroid gland can be evaluated by several imaging techniques. One is radionuclide imaging, which is discussed in detail in Chapter 12. The others are plain radiography, ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI). Each has advantages and limitations, and, with the exception of US in patients with a detected thyroid nodule, there is no absolute clinical indication for performing them in the majority of patients (2). The major limitation of all the techniques, in addition to expense, is their lack of specificity for tissue diagnosis. This chapter will focus on the clinical use of US, CT, and MRI, and as far as possible compare their advantages and disadvantages (Table 14.1).
Plain Radiography
Ultrasonography
Ultrasonographic examination of the neck is performed using high-frequency transducers (7 to 15 MHz) with the patient in the supine position and the neck hyperextended. The transducer is coupled to the skin with gel because the sound waves do not pass through air. US can detect thyroid lobes or lesions as small as 1 to 2 mm. It can distinguish solid nodules from simple and complex cysts. It allows accurate estimation of thyroid size, gives a rough estimate of tissue density (echogenicity), shows vascular flow and velocity (color flow Doppler), and aids in the accurate placing of needles for diagnostic or therapeutic purposes (5). Finally, it allows in utero investigation of the fetal thyroid (6,7).
Table 14.1 Characteristics of Commonly Used Imaging Procedures in Relation to Disorders of the Thyroid | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
The major limitations of US are the high degree of observer dependency (8,9) and the inability to identify retrotracheal, retroclavicular, or intrathoracic extensions of the thyroid (3,5). Images are obtained in the transverse (axial) and longitudinal (sagittal) planes, and sometimes in the oblique planes. The procedure rarely takes more than 5 to 10 minutes.
US is based on the emission of high-frequency sound waves and their subsequent reflection as they pass through the tissue. The amplitude of the reflections of the sound waves varies according to differences in the acoustic impedance of the various tissues. Therefore, small calcifications of 1 mm with high acoustic impedance may be seen, whereas a large thyroid nodule with acoustic characteristics similar to that of normal thyroid tissue may not be seen. High-frequency sound waves penetrate tissue less well than do low-frequency waves, but the structural resolution of the high-frequency waves is better. The frequency used to visualize the thyroid is a compromise between the need for depth of penetration and that for resolution. The use of real time allows differentiation of static structures (thyroid, neck muscles, and lymph nodes) from moving or pulsating structures (blood vessels, esophagus).
Transducers equipped with color flow Doppler capabilities have made it possible to display the speed and direction of blood flow. This methodology encodes the frequency of pulsed Doppler signals by color, which are then overlaid on B-mode sonograms with their two-dimensional spatial information. Detection of velocities of less than 1 cm/s, in combination with increased spatial resolution (high-frequency US), allows visualization of very small vessels, so that the vascularity of small areas such as thyroid nodules can be assessed (10). The introduction of power Doppler US, displaying the strength of the Doppler signal in color, increases the gain (amplitude of the signal) by 10 to 15 dB, resulting in improved sensitivity for detection of flow (11). Contrast media for US can, in principle, increase the Doppler signals by up to 25 dB (12).
Developmental Abnormalities
US may aid in the diagnosis of thyroid agenesis or hypoplasia (Table 14.2) (13,14). Thyroglossal duct cysts as well as ectopic thyroid tissue represent the majority of congenital thyroid anomalies in the neck and may be identified by US (15,16).
The Normal Thyroid Gland
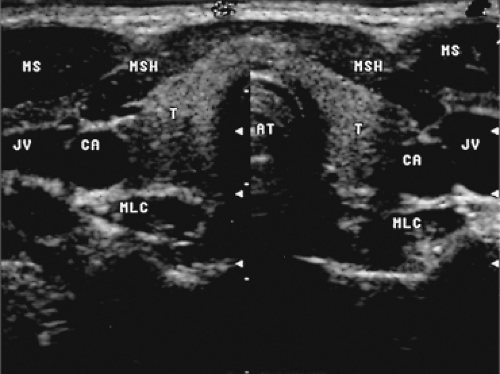
Normal thyroid parenchyma has a characteristic homogeneous medium-level echogenicity (Fig. 14.2), whereas that of the muscles anterior and anterolateral to the thyroid is lower (17). Posterolaterally, the thyroid is bordered by the sonolucent common carotid artery and internal jugular vein, and medially by the trachea. The esophagus with its echogenic mucosa can usually be seen behind and to the left of the trachea.
A high proportion of subjects with a normal thyroid gland have small (<1 cm in diameter) cystic or solid nodules, often
called “incidentalomas.” Their frequency is higher in women, increases with age, and varies between countries, possibly related to differences in iodine intake (see Chapter 49) (18). The importance of these abnormalities is unclear, but because these incidental sonographic nodules are common, whereas thyroid carcinoma is not, a conservative approach is usually recommended (19). Biopsy, however is generally indicated for nodules >1 cm in diameter with two or more suspicious findings (e.g., taller-than-wide shape, marked hypoechogenicity, microcalcifications, poorly marginated nodule) on US (20,21).
called “incidentalomas.” Their frequency is higher in women, increases with age, and varies between countries, possibly related to differences in iodine intake (see Chapter 49) (18). The importance of these abnormalities is unclear, but because these incidental sonographic nodules are common, whereas thyroid carcinoma is not, a conservative approach is usually recommended (19). Biopsy, however is generally indicated for nodules >1 cm in diameter with two or more suspicious findings (e.g., taller-than-wide shape, marked hypoechogenicity, microcalcifications, poorly marginated nodule) on US (20,21).
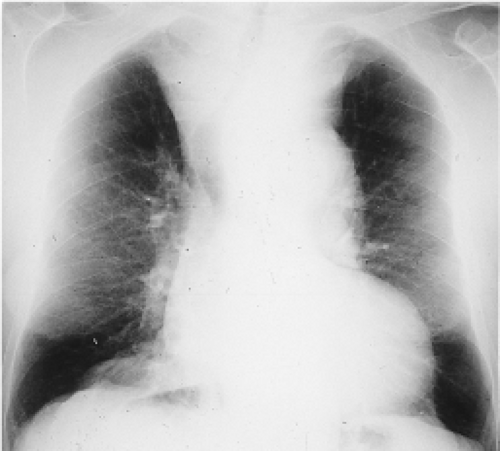 Figure 14.1 Chest radiograph showing a large mediastinal goiter causing tracheal deviation to the right and tracheal compression. |
Table 14.2 Possible Applications of Ultrasound in Patients with Thyroid Disorders | |
|---|---|
|
Goiter (i.e., an enlarged thyroid gland) is a general diagnosis based on physical examination, but the average error of this examination is approximately 40% and increases with thyroid size, and therefore it cannot be used to determine thyroid size reliably (22). Two sonographic methods for quantitating thyroid size are available. One is based on the volume of an ellipsoid, with the formula length × width × thickness × π/6 for each lobe. This method is 80% to 85% accurate, the accuracy decreasing with increasing size and degree of irregularity of the thyroid (23). The other method is based on obtaining cross-sectional images of the entire thyroid gland; its accuracy is 90% to 95%, and it is less influenced by size and degree of irregularity (24). The accuracy and precision of thyroid-volume determination using three-dimensional US (multiplanar approximation) is currently being evaluated (25). In normal subjects the thyroid volume (5 to 20 mL in adults) is under genetic control (26). However, it is also positively related to body weight and age and influenced by physiologic as well as environmental factors; for example, it is increased by low iodine intake and smoking (27,28,29). US is the most sensitive technique for screening for goiter and is widely used for this purpose in field studies (30).
Diffuse Thyroid Disease
Non-autoimmune nontoxic diffuse goiters appear on US as diffusely enlarged thyroid lobes with a uniform or discretely irregular echo pattern. Various degrees of hypoechogenicity may be evident, but marked hypoechogenicity suggests the presence of goitrous autoimmune thyroiditis (Hashimoto’s thyroiditis) (31,32). In the latter, the hypoechogenicity is not only marked but may be inhomogeneous (Fig. 14.3) (31,32). US cannot differentiate between goitrous autoimmune thyroiditis and lymphoma. Therefore, growth of a goiter, especially in patients with autoimmune thyroiditis receiving thyroxine (T4) therapy, should raise suspicion of lymphoma and lead to biopsy (33). The appearance of multinodular goiters also may mimic that of goitrous autoimmune thyroiditis.
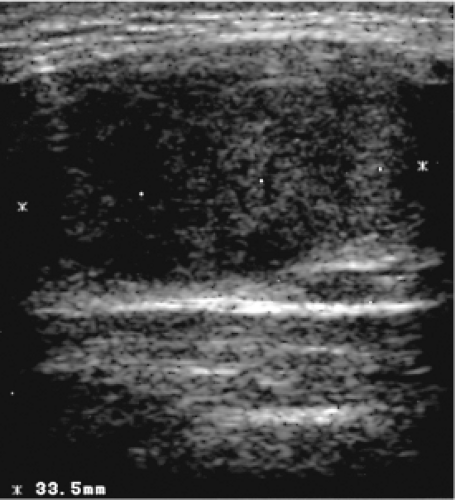 Figure 14.3 Longitudinal sonogram showing diffuse hypoechogenicity in a patient with goitrous autoimmune thyroiditis (Hashimoto’s thyroiditis). |
In patients with Graves’ thyrotoxicosis, the thyroid is usually enlarged and the echo pattern is homogeneous, but it may be nodular in patients with long-standing disease. Echogenicity is normal to markedly decreased. There is debate as to whether marked hypoechogenicity at the time of cessation of antithyroid drug therapy is a marker for recurrence of thyrotoxicosis (34). Color Doppler US reveals rich vascularity and increased flow (the so-called “thyroid inferno”), which are correlated with the degree of thyroid hyperfunction. Among patients with Graves’ thyrotoxicosis, blood flow in the thyroid artery may be higher in those patients who subsequently have recurrent thyrotoxicosis than in those who remain euthyroid after antithyroid drug therapy is discontinued (35). In contrast, blood flow is decreased in patients with exogenous thyrotoxicosis, allowing rapid distinction between them and patients with Graves’ disease (36). Among patients with amiodarone-induced thyrotoxicosis, color Doppler US may distinguish between those with iodine-induced thyrotoxicosis (type I), in whom thyroid vascularity is abundant, and those with thyroiditis (type II), in whom the thyroid is avascular and in whom glucocorticoid treatment is effective (see section on effect of excess iodine in Chapter 10) (37). Subacute granulomatous thyroiditis results in thyroid enlargement and areas of hypoechogenicity, probably related to areas that are affected by the inflammatory process (38), and vascularity is decreased (39). With recovery, size decreases, but areas of hypoechogenicity may be detected for many months (38). Color Doppler US distinguishes patients with the multinodular variant of Graves’ disease (extranodular diffuse hypoechogenicity with increased color Doppler signal and maximal peak systolic velocity) from those with non-autoimmune toxic multinodular goiter (normal extranodular vascularity) (40).
Multinodular Goiter
Multinodular goiters are usually larger than diffuse goiters, and 10% to 20% have a substernal or intrathoracic extension that cannot be visualized by US because the bony thorax prevents penetration of sound waves (41). The echographic structure of multinodular goiters may be heterogeneous without well-defined nodules, or there may be multiple nodules interspersed throughout a normal-appearing gland. Areas of hemorrhage, necrosis, and calcifications are often seen. These goiters, as noted above, may be diffusely hypoechogenic and therefore difficult to distinguish from goitrous autoimmune thyroiditis.
The majority of patients evaluated for a solitary nodule have additional small thyroid nodules detected by US (41). The echogenicity of the nodules varies from hyper- to iso- to hypoechoic, often even in the same patient. The presence of multiple nodules, as detected by US or any other imaging procedure, does not exclude carcinoma, and indeed it is just as likely to be present in a multinodular goiter as in a solitary nodule (42,43,44). Therefore, especially in view of the increasing use of nonsurgical treatment for multinodular goiter (see Chapter 48) (41), fine-needle aspiration (FNA) biopsy is usually indicated, especially in euthyroid patients with a dominant or a growing nodule. US guidance, rather than palpation guidance, is recommended because it facilitates sampling of nodular lesions and is associated with a lower rate of false-negative results (see Chapter 49) (45).
Table 14.3 Ultrasonography Criteria Assessing Risk of Thyroid Nodule Malignancy | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Thyroid Cysts
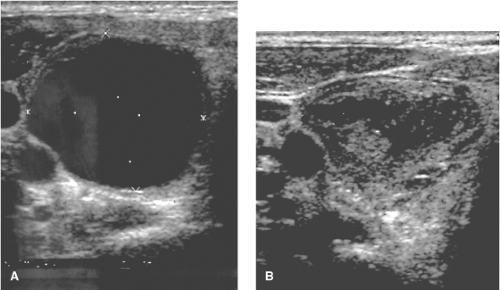
Thyroid cysts are well-defined areas with greatly reduced or no echogenicity (Fig. 14.4), but there may be a few echoes if the cyst contains debris, microcrystals, or necrotic tissue. True simple cysts are rare (perhaps 1% of all nodules) and virtually always benign (41,46). More often, cysts are complex or mixed, with both cystic and solid components. A “honeycomb” or “spongiform” appearance, multiple tiny cystic structures separated by thin septations in more than 50% of the nodule, is a feature with a high positive predictive value for benignity (46,47). The prevalence of cancer in nodules that are >50% cystic is low (48), and recent guidelines recommend that such nodules should not be aspirated unless it is for therapy, rather than diagnosis (21). The risk of initial nondiagnostic cytology is largely predicted by the presence of a cystic component of the nodule (49). After US-guided aspiration, the residual solid component should be examined via biopsy (Fig. 14.4). If the cytology is benign and the cyst recurs, as it does in approximately 50% of patients (41), US-guided treatment can
be offered. Injection of tetracycline is not effective in preventing further recurrence (50), but injection of ethanol is effective (51). Combined aspiration and subsequent interstitial laser therapy also seems promising (52).
be offered. Injection of tetracycline is not effective in preventing further recurrence (50), but injection of ethanol is effective (51). Combined aspiration and subsequent interstitial laser therapy also seems promising (52).
Benign Thyroid Nodules
In unison, recent guidelines suggest that a thyroid US should be performed in all patients with known or suspected nodular thyroid disease and also used as a screening tool in high-risk patients (e.g., history of childhood neck radiation, family history of MTC and MEN 2) (20,21,53). However, most benign thyroid nodules (thyroid adenomas, hyperplastic nodules) are hypoechoic relative to normal thyroid tissue, but so are thyroid carcinomas, and the two cannot be distinguished reliably on the basis of size, degree of echogenicity, or presence of a sonographic halo, calcifications, or vascularization (54). Table 14.3 summarizes low-risk versus high-risk features found on US (53). The coexistence of two or more suspicious criteria on US greatly increases the risk of thyroid cancer (20). Real-time US elastography is a newly developed technique to study the hardness/elasticity of nodules to differentiate malignant from benign nodules (55,56). Tissue displacement is displayed using a color scale usually ranging from red (highest elasticity corresponding to lowest risk of malignancy) to blue (lowest elasticity and corresponding to highest risk of malignancy). Its use is limited in smaller nodules, cystic nodules, and if coarse calcifications are present (46), and the technique requires further evaluation (57).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree