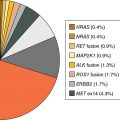
Learning Objectives: 1. What is oligometastatic non–small cell lung cancer (NSCLC)? 2. What clinicopathological features are suggestive of oligometastatic disease? 3. How does the eighth edition of the TNM (tumor, node, metastasis) staging system account for this concept? 4. How can the clinician determine whether 2 foci of lung cancer represent metastatic disease or independent primary tumors? 5. What is the ideal treatment approach and extent of resection in this subgroup of patients? Most patients with advanced NSCLC receive systemic therapies as primary treatment, with therapeutic goals focused on palliation. However, it is becoming increasingly evident that patients with stage III-IV NSCLC are a heterogeneous group in regard to disease burden and prognosis. In its eighth edition, the TNM staging system was updated to reflect the diversity in this patient population, increasing our capacity to refine prognosis. Radiologic and therapeutic advances such as positron emission tomography (PET) and immunotherapy have improved survival for many patients with advanced lung cancer and led some to reconsider surgical intervention with curative intent for select patients with advanced NSCLC.1,2 In this chapter, we define oligometastatic disease, summarize the current literature regarding the role of surgical resection, and discuss key considerations in the surgical management of patients with suspected oligometastatic NSCLC. The term oligometastatic disease was first introduced by Hellman and Weichselbaum in 1995 to describe patients with a more indolent tumor biology and limited number of metastases that could be amenable to cure by means of local surgical therapies.3 “In these cases the slow progression of the malignancy raises the opportunity for an aggressive local approach to control the disease” (page 9). While no clear consensus on the criteria defining oligometastatic disease has been reached, the patients most often included in this category are those with 1-5 metastases, locally advanced stage III, and metachronous second primary lung cancer (SPLC). There is growing evidence to suggest that oligometastatic lung cancer is a biologically and clinically distinct entity, which may redefine the prognosis and treatment approaches for many patients with historically incurable disease. Several studies have demonstrated long-term survival in certain patients with stage III and IV NSCLC following definitive surgical resection of both locoregional and distant metastases. Early evidence is, however, based almost exclusively on retrospective series, limiting conclusions on which patients benefit most from surgery and the most effective treatment approaches. As our understanding of oligometastatic NSCLC improves, characterization of predictable and reliable prognostic factors may facilitate proper patient selection. Identifying patients with oligometastatic disease is clinically challenging. Many patients presenting with apparent oligometastatic lung cancer progress to overtly metastatic disease. Objective predictors of the rate of metastatic progression are necessary to improve selection of patients who may achieve long-term disease control with aggressive oligometastasis-directed surgical therapy. Ashworth et al. conducted a meta-analysis characterizing outcomes and prognostic factors associated with oligometastatic stage IV NSCLC. The meta-analysis involved 757 patients with 1-5 metastases undergoing surgical resection of all lesions with curative intent. Median and 5-year overall survival (OS) were 26 months and 29.4%, respectively, while 8-year OS was 23.4%.4 Low-risk patients, defined as those with metachronous metastases and any N stage, exhibited 47.8% 5-year OS, compared to 36.2% and 13.8% for intermediate-risk (synchronous metastases, N0) and high-risk (synchronous, N1-2) patients, respectively.4 Here and for the purpose of this chapter, metachronous disease is diagnosed at least 2 months after the primary tumor, while synchronous disease is of course discovered concurrently. Significant factors portending long-term survival include R0 resection of the primary tumor, a long disease-free interval with metachronous rather than synchronous presentation, negative mediastinal nodal status, adenocarcinoma histology, and metastases limited in number and organ sites.1,4,5 Lussier et al. compared microRNA expression of lung metastasis specimens resected with curative intent between patients with high and low rates of recurrence. Several prioritized microRNAs were identified that consistently distinguished a high from a low rate of metastatic progression and OS.6,7 Interestingly, this pattern of microRNA expression was not found in primary tumor specimens, which may be attributed to high genetic heterogeneity within the primary tumor and clonal selection within metastatic sites.6 Further investigation of a molecular basis for tumor phenotype in NSCLC may aid in identification of patients most likely to experience long-term disease-free survival following surgical resection. The most common sites of metastasis in NSCLC are lung, brain, adrenal glands, and bone. Intracranial lesions are found in 25%-35% of patients at the time of NSCLC diagnosis.8 Retrospective studies have reported long-term survival in patients with solitary brain metastases following surgical treatment of both locoregional and intracranial disease.9–11 A recent Surveillance Epidemiology and End Results (SEER) Program database study of the survival impact of combined surgical resection of the primary tumor and oligometastatic disease in 220 patients with brain-only M1 disease demonstrated combined surgery was associated with improved OS (p < .0001).11 A Mayo Clinic study published in 2001 showed that surgical resection of synchronous brain metastases and primary NSCLC resulted in significantly superior 5-year survival in patients with N0 disease compared to those with N1/2 disease, with median OS of 44 months versus 10 months, respectively.12 In their latest edition of clinical practice guidelines, the American College of Chest Physicians (ACCP) recommended resection or radiosurgical ablation in patients with metachronous single brain metastasis as well as those presenting with synchronous brain metastasis and resectable N0-1 primary NSCLC.13 In most instances, the preferred strategy is surgical or ablative therapy of brain metastases prior to lung resection given the clinical levity of neurological symptoms due to mass effect (Figure 15-1). Figure 15-1. Suggested algorithm for surgical management of NSCLC with extrathoracic oligometastasis. Only the most common organ sites are included. Chemotherapy and radiation have been omitted, as has the management of frankly metastatic states indicated in italics. The adrenal glands are the second most common extrathoracic site of NSCLC metastasis. A systematic review of isolated adrenal metastasis published in 2008 again showed patients with synchronous disease had a shorter median OS compared to those with metachronous disease, yet had equivalent 5-year survival estimates of 26% and 25%, respectively.14 A study by Barone et al. found that median OS time of patients who underwent adrenalectomy was 31 months, likened to 13 months for patients who only underwent medical treatment. It is of no surprise that OS was significantly worse for bilateral than ipsilateral or contralateral metastasis (11 months vs. 27 months vs. 29 months, respectively).15 The ACCP recommends resection of isolated metachronous and N0,1 synchronous adrenal metastases along with the primary tumor given negative invasive mediastinal staging (Figure 15-1).13 Recent anatomical evidence of direct lymphatic channels between the chest and adrenal glands suggests that metastatic disease in the adrenal glands may represent locoregional lymphatic spread rather than hematogenous metastasis.16 Bone metastases carry a dismal prognosis associated with substantial morbidity and mortality. Poor outcomes are repeatedly reported despite surgical resection and radiotherapy, with median OS ranging from 0 to 13 months.10,17,18 As a result, it may be argued that bony metastasis can seldom, if ever, be considered oligometastatic disease in the setting of an NSCLC primary. The incidence of metachronous and synchronous lung cancer is increasing due to advances in early detection and treatment in recent years. The management of patients with multiple pulmonary lesions suspicious for lung cancer is clinically challenging and raises many unique issues to consider. Take, for instance, the patient with potentially operable lung cancer found to have an additional small lesion on imaging. A great majority of additional pulmonary nodules with typical computed tomographic (CT) appearance are benign and should be surveilled according to published guidelines irrespective of accompanying malignancy.19,20 The diagnostic dilemma is determining whether 2 foci of lung cancer represent metastatic disease or independent primary tumors. Clinicians traditionally have relied on criteria proposed by Martini and Melamed in 1975 to help make this distinction.21 SPLCs may be identified histologically if a different cell type than the primary tumor is found; however, two thirds of SPLCs reported in the literature have been the same cell type as the primary cancer.13 Some authors suggested primary NSCLCs may be distinguished on the basis of the most dominant histological subtype (papillary, acinar, etc.) and a variety of cytologic and stromal features.22 There has been recent interest in utilizing genetic and molecular characterization to differentiate SPLCs and pulmonary metastases on the basis of tumor clonality.23,24 Liu et al. examined the clonal relationship between lung tumors in several patients with multiple synchronous pulmonary lesions meeting 2007 ACCP criteria for hematogenous or intrapulmonary metastases using whole-genome and exome sequencing. The authors found that despite their clinicopathological classification, tumors harboring shared mutations in the same patient were extraordinarily rare. Such genetic heterogeneity suggests multiple primary tumors may be widely underdiagnosed.24 While whole-genome sequencing and whole-exome sequencing of every lung tumor is not clinically practical, several studies have reported limited molecular analysis of cancer-related genes such as EGFR (epidermal growth factor receptor), P53, and ERBB2, with mixed results.23,25 Tumor histology and genetic characterization have been challenged on theoretical grounds based on the tendency of malignant neoplasms to acquire mutations, intratumoral genetic heterogeneity, and even the ability to transform from adenocarcinoma to small cell lung cancer.26,27 While not to be regarded as empirically definitive alone, these features should be taken into account and considered alongside the spatial and temporal relationship of the 2 foci of cancer, as well as the mediastinal nodal status. Furthermore, concordance of nodal status, histology, anatomy, and timing may be most indicative of a diagnosis. For instance, pulmonary lesions of identical histology in a patient with multiple systemic metastases may suggest pulmonary metastases, as do histologically identical tumors in different lobes with N2-3 involvement.13,28 An interval less than 2 years between occurrences of lung cancer suggests pulmonary metastasis, while a metachronous interval greater than 4 years between occurrences favors multiple primary lung cancers.13,29 Tumors of the same histology in different lobes may be considered independent primary cancers in the absence of N2,3 nodal involvement and systemic/extrathoracic metastases. A second lesion of different histology arising from a nearby focus of carcinoma in situ is suggestive of multiple primary lung cancers.13,29 Given the number of factors at play, it is critical that cases be discussed in a multidisciplinary setting involving an experienced radiologist, pathologist, oncologist, and thoracic surgeon, as this distinction will influence the prognosis and management of these patients. Multiple primary lung cancers should be staged independently and managed as distinct tumors. Surgery is the treatment modality of choice for patients with multiple NSCLCs, if feasible. Pooled 5-year OS after resection of metachronous second primary NSCLC ranges from 41% to 46% in meta-analyses13,30 and is as high as 54.5%-77% in recent series; however, a majority of patients included in the latter studies had stage I disease.31–35 Anatomic resection of the second tumor is preferred in most studies, with the remaining 30%-40% of patients undergoing segmentectomy or wedge resection.13,30 A handful of series have reported survival in regard to extent of resection, with mixed results.31,32,34 Despite the association of limited resection with locoregional recurrence, OS rates are not significantly different after wedge resection or segmentectomy compared to lobectomy in most studies, and sublobar resection is an acceptable alternative for patients with limited pulmonary reserve unable to tolerate more extensive resection.31,35,36 It may be argued that segmentectomy or wedge resection is the most appropriate approach for early stage metachronous primary tumors in such patients. There is some recent evidence to suggest that segmentectomy is associated with lower recurrence as well as better progression-free and OS than wedge resection for early stage NSCLC, but this has not been evaluated in patients with oligometastatic disease.37,38 The eighth edition of the AJCC staging system subclassified stage IV disease into M1a, M1b (single extrathoracic metastasis in solitary organ), and M1c (multiple metastases in a single organ or in multiple organs), with median OS of 22.5, 17.8, and 13.6 months, respectively (p < .001).39 Patients with pulmonary metastases typically undergo multimodal treatment, with more favorable outcomes compared to those with extrathoracic metastases. Patients with contralateral pulmonary metastases exhibited superior OS compared to patients with pleural/pericardial M1a disease or extrathoracic metastasis, but inferior OS compared to those with multiple primary lung cancers.18,39,40 In a SEER database study, Morris et al. examined the survival impact of a contralateral tumor nodule with that of regionally advanced contralateral N3 involvement. Compared to N3M0 disease, N0-1M1a patients with contralateral pulmonary metastasis experienced superior OS for primary tumors 2 cm or less (T1b), equivalent survival for primary tumors 2-7 cm (T1c-T3), and inferior survival for those 7 cm or greater (T4). This suggests that T stage of the primary tumor may carry greater prognostic significance in patients with intrapulmonary metastases than those with advanced locoregional disease, as the former are subject to multiple pulmonary resections. Survival outcomes of intrapulmonary M1a patients in this study were not significantly different from patients of comparable T and N stages with a second early stage primary lung cancer. This may reflect difficulty differentiating multiple primary lung cancers from metastatic disease from pooled retrospective data.40 Few studies have investigated the survival impact of surgery for stage IV NSCLC with isolated pulmonary metastasis. The 5-year OS following definitive surgery is 22.5%-48.5%.18,41 Patients with N0-1 and metachronous pulmonary metastases who underwent complete R0 resection of the primary tumor had the most favorable long-term outcomes.4,18 Invasive mediastinal staging with video-assisted mediastinoscopy is essential prior to surgical intervention, even with negative CT/PET imaging.42 A staged approach, including lobectomy of the primary tumor with systematic lymph node dissection followed by limited resection of contralateral metastases, may be appropriate for patients with multiple pulmonary lesions.18,43 Systematic mediastinal lymph node dissection (MLND) has been shown in several randomized clinical trials to be far superior to selective lymph node sampling and no different from complete MLND in identifying positive N2 nodal stations.44 The goals of surgery should be curative, with emphasis on complete resection of the primary tumor, as well as adequate mediastinal lymph node harvesting (Figure 15-2). Figure 15-2. Suggested algorithm for surgical management of NSCLC with contralateral pulmonary lesion. A second primary lung cancer may less frequently present in a synchronous manner. SBRT, stereotactic body radiation therapy. The management of stage IIIA-IIIB-N2 NSCLC is one of the most controversial topics in thoracic oncology despite results from a handful of phase 3 randomized trials having been available for years. Although N2 nodal disease typically has negative prognostic implications in terms of long-term survival, a subset of patients with stage IIIA-N2 lung cancer may benefit from surgery as part of multimodal therapy. The eighth edition of the AJCC staging system subclassifies N2 disease into N2a1 (single ipsilateral mediastinal or subcarinal nodal station without pN1 involvement, ie, skip N2); N2a2 (single pN2 nodal station with pN1 involvement); and N2b (multiple pN2 nodal stations involved). Corresponding 5-year survival rates following definitive surgery are 52%, 41%, and 36%, respectively (p < .01).45 Survival outcomes of N2a1 patients are not significantly different from those with only N1b disease.45 Two recent studies indicated that the number of metastatic N2 lymph nodes and total metastatic lymph node ratio are independent predictive factors of disease-free survival.46,47 Utilization of validated invasive mediastinal staging techniques is essential to provide patients every opportunity for long-term survival. There is roughly a 20% chance of N2 nodal station involvement in the setting of negative CT or PET imaging of the mediastinum.44 Complete restaging after induction therapy is crucial in guiding subsequent treatment and has been shown to improve OS independently. This should include invasive mediastinal staging, head CT magnetic resonance imaging (MRI), plus either whole-body PET or abdominal CT and a bone scan.13 One of the dilemmas in management of patients with oligometastatic N2 disease is the timing of surgery and systemic therapy, as one modality of treatment may affect the patient’s tolerance of the other. Advantages of upfront surgery include the procurement of tissue to guide systemic therapy and timely control of the primary tumor. Treatment-naïve patients have preserved functional status and tend to tolerate surgery better in general. Benefits of neoadjuvant therapy followed by surgery include early initiation and superior compliance with systemic therapy. Some advocate a period of neoadjuvant therapy in the setting of N2 disease to gauge tumors’ metastatic potential, reserving surgery for patients with more favorable tumor biology who demonstrate some degree of mediastinal clearance (Figure 15-3).48,49 Furthermore, patients who do not constitutionally tolerate chemotherapy may be less likely to tolerate an aggressive operation.50 Figure 15-3. Suggested algorithm for surgical management of locally advanced NSCLC. Three relevant phase 3 randomized clinical trials have been conducted in patients with stage IIIA-N2 lung cancer; however, they did not provide definitive evidence regarding the optimal treatment approach.51–53 In the Intergroup and ESPATUE trials, patients underwent induction chemoradiotherapy before being randomized to either surgery or further radiotherapy; patients in the ESPATUE trial also received platinum-based chemotherapy concurrently with chemoradiation prior to randomization. In the European Organization for Research and Treatment of Cancer (EORTC) trial, patients received induction platinum-based chemotherapy alone; those who responded were randomized to surgery or radiotherapy. OS was not significantly different between arms in any trial, although progression-free survival (PFS) in the Intergroup trial was superior in the surgery arm. Of note, 44% of patients in this study underwent pneumonectomy, with unacceptable perioperative mortality. A matched subgroup analysis excluding pneumonectomy found a significant survival difference favoring neoadjuvant chemo-radiation therapy (CRT) plus lobectomy over chemoradiation alone, with median and 5-year survival of 33.6 versus 21.7 months and 36.1% versus 17.8%, respectively (p < .002).52 The best long-term results may be obtained with proven mediastinal clearance or downstaging, when a lobectomy is feasible to achieve R0 resection (Figure 15-3).52,54 The evidence of survival benefit associated with surgery is much stronger in studies of trimodal therapy than bimodal therapy, reflecting the synergistic effects of metastasis source control, ablation of locoregional micrometastatic lymph node disease, and systemic chemotherapy.55 Another subset of patients is found to have pN2 disease at the time of surgery for cN1 or clinically node-negative cancer (cN0). Patients typically undergo adjuvant chemotherapy with or without radiation therapy, with 5-year survival rates ranging from 30% to 47%.56,57 This has been described as incidental, unsuspected, ignored, or underappreciated pN2 disease, depending on the clinical circumstances and extent of preoperative staging.58 This is essentially the only group of patients with stage III-N2 lung cancer commonly treated with upfront surgery in the current era of systemic therapies. Most evidence suggests a clear survival benefit of mediastinal downstaging prior to surgery for N2 disease (Figure 15-3). However, recent studies of patients upstaged to pN2 at the time of surgery and given adjuvant therapy have failed to show a difference in survival compared to patients receiving neoadjuvant therapy prior to surgery.57,58 Similarly, a recent comparison of neoadjuvant and adjuvant chemotherapy alone in combination with surgery for stage III-N2 disease demonstrated no survival difference.56 Again, a wide range of survival outcomes and conflicting findings reflect a high degree of heterogeneity among N2 patients and further necessitates ongoing randomized prospective trials with standardized chemo- and radiation therapy regimens. As diagnostic and therapeutic advances improve survival of patients with NSCLC, we can expect to encounter more patients with oligometastatic disease. Large randomized trials with standardized systemic therapy regimens and patient selection criteria are needed to further investigate surgical management of oligometastatic NSCLC. Thorough staging and restaging prior to intervention and judicious patient selection based on validation of clinical and biological prognostic indicators will enhance delivery of appropriate treatment to this complex group of patients. While controversial, a definitive surgical approach appears to be associated with favorable long-term outcomes in select patients with advanced NSCLC undergoing multimodal therapy. A 69-year-old female presents to the emergency room with complaint of lower back pain. She has a 45 pack-year smoking history, but no other significant past medical history and no trauma. Lumbar spine CT shows a 1.4-cm lytic lesion in the L4 vertebral body. Chest, abdomen, and pelvis CT show a 3.6-cm left upper lobe lesion with associated mediastinal adenopathy. The brain MRI shows a 1.2-cm left frontal lobe lesion concerning for metastasis. The spine biopsy is positive for adenocarcinoma and is TTF-1 positive. Learning Objectives: 1. The incidence of bone metastases in NSCLC is discussed. 2. Risks, benefits, and rationale of radiotherapy in the palliative setting are examined. 3. Understanding the emerging indications for local therapy, including stereotactic body radiation therapy (SBRT), in the oligometastatic patient is delineated. 4. The incidence of brain metastases in NSCLC is discussed. 5. Elucidation of information for understanding the risk, benefit, and rationale of radiotherapy, both stereotactic radiosurgery (SRS) and whole-brain radiotherapy (WBRT), for central nervous system (CNS) metastases is given. Bone metastases are a common sequela of NSCLC and can become very debilitating secondary to pain, pathologic fracture, and spinal cord compression. Bone metastases are becoming more common as patients live longer with cancer and imaging becomes more sensitive and aggressively used. Studies have shown bone metastases in 5%-30% of patients with lung cancer.59 There is high-level evidence that external beam radiotherapy (EBRT) can be effective and safe in treating bone metastases. Multiple studies have shown a significant improvement in pain with both fractionated and single-fraction EBRT. Studies suggested that 53%-88% of patients can expect at least partial response for pain, while 17%-24% can expect complete resolution of symptoms.60–63 A meta-analysis by Chow et al. analyzed 25 randomized trials including 5,617 patients and reported a 60%-61% response rate.61 Of patients with some response, 70% noticed pain relief within 2 weeks and 90% noticed within 2 months.61 Traditional palliative radiotherapy courses for bone metastases include 8 Gy in 1 fraction, 20 Gy in 5 fractions, 24 Gy in 6 fractions, and 30 Gy in 10 fractions, among others. These have been compared in multiple studies. Chow et al. showed that single-fraction and multifraction treatments did not show a difference in any response (60% vs. 61%) or complete response (23% vs. 24%).61 Also, 8 Gy in 1 fraction was found to be equivalent to 24 Gy in 6 fractions (response rate 53% vs. 56%).63 Re-treatment rates were increased for patients receiving single-fraction treatment versus a multifraction approach (20% vs. 8%61; 13.3% vs. 8.8%60; 15% vs. 5%62). Treatment for bone metastases is minimally toxic but can present issues depending on the location of treatment. Specifically, when considering if fractionation affects toxicity, Majumder et al. showed equivalent toxicity rates in patients receiving 30 Gy in 10 fractions versus 8 Gy in 1 fraction to vertebral body metastases.64 Chow et al. also showed equivalent rates of pathologic fracture and spinal cord compression between the 2 groups.61 A well-recognized side effect of palliative radiotherapy for bone metastases is the pain flare, or a temporary worsening in pain at the treatment site shortly after radiotherapy. It is theorized that cytokine release is responsible and can be seen in 30%-40% of patients.65 A phase 3 study randomizing patients to prophylactic dexamethasone (8 mg 1 hour before treatment and the following 4 days) versus placebo showed a reduced frequency of pain flare with prophylactic steroids (26% vs. 35%, p = .05). In the dexamethasone arm, 3/148 patients had a hyperglycemic event. The duration of pain flare was 3 days with dexamethasone and 2 days with placebo.66 While this study supported prophylactic dexamethasone, the adoption of this as a standard of care is debated secondary to dexamethasone toxicities and the relatively limited clinical improvement.67 According to the most recent American Society for Radiation Oncology (ASTRO) bone metastases guidelines, patients with persistent or recurrent pain 1 month after palliative treatment are eligible for re-treatment.68 A meta-analysis showed that 58% of patients who received retreatment will have a response to radiotherapy. The majority of these patients received single-fraction therapy, both primarily and in the re-treatment setting. Of these patients, 23% had lung primary cancer.69 There is high-level evidence that radiotherapy is safe and effective for bone metastases. Patients can be treated with single-fraction or multifraction regimens with similar responses and toxicity. Toxicity is limited and corresponds to treatment area. Pain flares are common and can be treated with dexamethasone. Re-treatment is possible and effective. The treatment algorithm for vertebral body metastases has become increasingly complicated with more advanced surgical and radiotherapy techniques. Memorial Sloan Kettering developed a systematic framework for decision-making: neurologic, oncologic, mechanical instability, and systemic disease, or NOMS.70 The following discussion provides details about each. It is important to note that neurologic and oncologic effects are considered together to determine the appropriate treatment option (Figure 15-4). Figure 15-4. Treatment algorithm for the management of vertebral body metastases. CEBRT, conventional external-beam radiation. (Reproduced with permission from Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18(6):744-751. © 2013 AlphaMed Press.) The neurologic consideration is determining the amount of epidural spinal cord compression (ESCC) present. The Spine Oncology Study Group developed a 6-point, MRI-based grading system for ESCC (Figure 15-5).71 Using axial T2-weighted images at the level of greatest compression, a score is given. Scores 0-1b are considered for radiation, scores of 2-3 are considered for surgery unless the tumor is of a radiosensitive histology (see oncologic consideration next). Score 1c treatment is not fully defined but may be amenable to SRS or separation surgery followed by radiation.70 Figure 15-5. Spine Oncology Study Group MRI-based grading system for ESCC. (Reproduced with permission from Bilsky MH, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324-328.) The oncologic consideration is essentially deciding the radiosensitivity of the tumor, which is based on the response to conventional EBRT. Traditionally, NSCLC has been considered an unfavorable, radioresistant histology along with melanoma and renal cell carcinoma. Lymphoma, seminoma, and myeloma are radiosensitive. Radiosensitive tumors can be treated with radiotherapy regardless of the ESCC score. Radioresistant tumors should follow the treatment algorithm based on the point score presented previously in this section.70 The Spine Oncology Study Group published an evidence-based system to determine the Spinal Instability in Neoplastic Disease, or SINS, score (Table 15-1). SINS is a point-based, objective measurement system using the spinal location, presence of pain, bone lesion appearance (blastic/mixed/lytic), radiographic alignment of the vertebral body, amount of vertebral body collapse, and amount of posterior involvement. Patients whose metastasis scores a 7-13 represent those patients with a potentially unstable lesion, and scores of 13 or greater represent an unstable lesion.72 All patients scoring 7 or greater warrant neurosurgical assessment. A 2014 study of the SINS score showed nearly identical intraobserver and interobserver agreement, confirming that it was reliable and reproducible.73 TABLE 15-1 Spinal Instability in Neoplastic Disease Systemic assessment is determining the patient’s ability to tolerate intervention, particularly paying attention to tumor burden, aggressiveness of disease, medical comorbidities, and the like. There are no hard-and-fast rules in this scenario, and a multidisciplinary discussion as well as the patient’s own goals should be considered.70 Spine metastases had been treated like other bone metastases, but it had been questioned if an increasing dose would lead to better, more durable pain control and fewer re-treatments. Conventional EBRT has limitations with dose escalation secondary to the sensitive structures near the vertebral bodies (ie, the spinal cord and esophagus). Stereotactic body radiation is highly conformal, allowing for safe dose escalation. Multiple phase 2 studies have shown that this is both safe and effective. Radiation Therapy Oncology Group (RTOG) 0631 is a phase 2/3 study where patients with a numerical rating pain scale of 5 or greater were treated with 16 Gy in 1 fraction. This showed that SBRT for spine lesions is feasible in terms of tumor coverage, normal tissue constraints, and toxicity.74 Phase 3 data, comparing 16-18 Gy in 1 fraction to 8 Gy in 1 fraction, are pending. Tseng et al. analyzed 279 de novo metastases in 145 patients receiving 24 Gy in 2 fractions. The 1- and 2-year local failure rates were 9.7% and 17.6%, respectively. No grade 3 toxicity was noted.75 Sprave et al. randomized patients to 24 Gy in 1 fraction versus 30 Gy in 10 fractions. There was no difference in pain relief at 3 months, although pain scores decreased faster with SBRT. SBRT also had greater pain relief at 6 months. No patients experienced grade 3 or higher toxicity.76 Contouring guidelines for SBRT alone77 and postoperative SBRT78 are available. Radionuclides or radiopharmaceuticals are radioactive, bone-seeking medications that can be used in patients with bone metastases. Through different mechanisms, these medications are selectively absorbed into metabolically active bone, thus delivering high doses of radiation to the diseased sites. Sumarium 153 is the most commonly used today, but other examples include strontium 89 and phosphorus 32. These are primarily used in breast and prostate cancers, but could be considered in the case of multifocal, painful bone metastases. Contraindications include severe marrow suppression and renal dysfunction. Oligometastatic disease is generally defined as a maximum of 5 metastatic lesions. Distinct categories include the following: 1. Synchronous: diagnosed within months of the primary tumor 2. Metachronous: appear after primary treatment Oligometastatic disease was previously considered incurable, but this treatment paradigm is now under question. Limited data on the benefit of local therapy are available, but studies have shown promising results. A propensity-matched analysis studying patients with synchronous metastases at the time of diagnosis showed a benefit to comprehensive local therapy. Inclusion criteria were pathologic confirmation of NSCLC; 1-3 metastases at time of diagnosis; radical treatment to metastases, including radiation or surgery; and completion of 2 cycles of induction chemotherapy or concurrent chemoradiation without disease progression. Exceptions were made for patients who had early intracranial treatment. There were 90 patients who met inclusion criteria. The majority had metastases that had non-squamous histology (89%) and had 1 metastasis (72%). Of the patients, 44/90 (49%) had metastases confined to the brain and 59% had brain metastases and metastases to other organs. Chemotherapy included a carboplatin-based doublet in 78% and a cisplatin-based doublet in 14%. Median number of chemotherapy cycles was 6. Out of 90 patients 69 received comprehensive local therapy (CLT). Median follow-up was 46.6 months. Cox proportional hazards regression analysis showed that patients who received CLT had a significant improvement in OS versus patients who did not (27.1 vs. 13.1 months). Univariate analysis showed that comprehensive local therapy, non-squamous histology (p = .03), T stage (p = .05), and favorable Eastern Cooperative Oncology Group (ECOG) performance status predicted for improved OS (p ≤ .01), although favorable ECOG was the only measure that remained significant in adjusted analysis. Non-brain/adrenal, single-organ metastases predicted increased PFS (p = .05) compared to brain/adrenal metastases. Eventually, 46/90 developed a new metastatic lesion, with 20 of those being in the brain. This study suggests that there is a subset of patients who would benefit from local therapy in the setting of oligometastatic NSCLC at diagnosis, particularly in patients with at least stable disease after primary therapy, limited metastases, and favorable performance status.79 Gomez et al. conducted a multicenter, randomized, phase 2 study in an attempt to define any benefit from aggressive local therapy in oligometastatic patients. Inclusion criteria were pathologically confirmed NSCLC stage IV disease according to the seventh edition of the AJCC guidelines, 3 or fewer metastases after first-line systemic therapy, ECOG less than 2, and standard chemotherapy (defined as 4 or more cycles of platinum doublet therapy, erlotinib/other first-line EGFR tyrosine kinase inhibitor [TKI] for 3 or more months if a patient had an EGFR mutation, or crizotinib for 3 or more months if the patient harbored an anaplastic lymphoma kinase [ALK] rearrangement). Patients with N1-N3 nodal involvement were considered to have 1 metastasis such that 2 additional extranodal metastases would qualify. Again, patients needing immediate treatment for CNS involvement were included in this study. Randomization included local therapy (surgery and/or radiation) and maintenance therapy versus maintenance therapy alone (which could be observation) in patients with 3 or fewer metastatic sites with stable/responsive disease after first-line systemic therapy. After systemic therapy, 74 patients were eligible for the study, of which 49 entered randomization. Twenty-five patients received consolidative local therapy, with 24 undergoing maintenance. Patient characteristics were comparable between the 2 groups. Most patients had adenocarcinoma histology and N0-N1 disease. A quarter of the patients had brain metastases. Of patients receiving local therapy, 48% were treated with radiation alone, 24% had surgery and radiation, 20% received chemoradiation, and 4% received surgery alone. In the maintenance group, 67% (16) received pemetrexed, 8% (2) erlotinib, 4% (1) afatinib, 4% (1) bevacizumab, and observation in 17% (4; 3 with squamous cell carcinoma [SCC] and 1 with sarcomatoid features). Median PFS in the local therapy arm was 11.93 months versus 3.9 months with maintenance therapy alone (p = .0054).80 Another potential benefit of radiotherapy in the setting of oligometastasis is the “abscopal effect,” or the ability of radiation to induce tumor regression in non-irradiated, distant tumor sites. This was first theorized in 1953.81 Patients with widely metastatic disease should have their cases discussed in a multidisciplinary setting. Radiotherapy is an important treatment option in this palliative setting. Brain metastases are diagnosed at presentation for 10% of patients with NSCLC,82 with the prevalence of intracranial disease as high as 26% for stage IV disease at presentation. The incidence of brain metastases varies with the molecular mutational profile of the primary tumor, with the rates of brain metastases higher in patients with EGFR mutations.83,84 Additionally, mutation status is prognostic for survival in NSCLC, with a median OS of almost 4 years for patients with adenocarcinoma and a graded pogostic assessment (GPA) of 3.5-4.0.85 Steroids should be promptly initiated for symptomatic patients with 4-32 mg of dexamethasone administered twice daily with a taper after maximal neurologic improvement.86 Surgical resection can be considered for patients with limited (1-3) brain metastases, lesions 3 cm or greater, controlled or absent systemic disease, and good performance status. Postoperative radiation should be delivered after surgical resection given high local recurrence rates with surgery alone.87 Definitive SRS is an effective and increasingly used treatment for patients with brain metastases, with local control rates greater than 80%. WBRT can be used to palliate patients with poor performance status or extensive intracranial disease. Best supportive care (BSC) may be considered for selected patients with poor performance status.88 Additionally, the development of targeted therapy for select patients with EGFR and ALK mutations has led to interest in deferring upfront radiation for limited, asymptomatic brain metastases. Pending level I evidence, caution may be warranted, with a multi-institutional retrospective suggesting upfront treatment with a first-generation EGFR TKI such as erlotinib with deferral of radiation therapy was associated with worsened survival.89 Multidisciplinary evaluation by providers, including thoracic surgeons, medical oncologists, and radiation oncologists, to determine optimal management for these patients is critical pending the results of prospective trials (NCT03497767). A 59-year-old Caucasian male with a past medical history of tobacco abuse, hypertension, chronic obstructive pulmonary disease (COPD), and multiple sclerosis currently on treatment presents with persistent abdominal pain and weight loss for 3 months. Chest CT shows a right lower lobe mass and hilar adenopathy. Abdomen/pelvis CT shows 2 liver lesions, one of which is biopsied and returns with a finding of squamous cell carcinoma. Brain MRI is negative for metastatic disease. He has no driver mutations, and the programmed death ligand 1 (PD-L1) value is 3%. Learning Objectives: 1. What basic molecular testing should be done for all metastatic NSCLC patients? 2. Which treatments should be avoided in squamous cell histology? 3. What are first-line treatment options for metastatic non-squamous NSCLC? 4. What are the first treatment options for metastatic squamous NSCLC? 5. Which agents are approved for continuation maintenance? 6. Which options are used for subsequent therapy? All patients with metastatic disease require systemic therapy, though cases of limited metastatic disease may have a multimodality approach, including radiation and surgery. Tissue biopsies should be sent for predictive biomarkers, such as EGFR, ALK, ROS1 (ROS proto-oncogene 1), BRAF, and PD-L1, all of which will help tailor treatment regimens. Of note, actionable mutations are more likely to be found in non-squamous and not otherwise specified (NOS) NSCLC than squamous histology. Though rare, ALK and EGFR mutations can be found in some squamous cell patients, especially those with no smoking history, small tissue sample, and mixed histology. More recently, additional predictive markers have emerged, namely, neurotrophic receptor tyrosine kinase (NTRK) gene fusions, ERBB2 (HER2), RET (REarranged during Transfection) gene rearrangements, MET amplifications, and tumor mutational burden (TMB), although there is still no consensus on the best way to measure some of these markers. The National Comprehensive Cancer Network (NCCN) panel suggested broad molecular testing in order to ensure identification of any driver mutations that may help guide therapies. Differing from these predictive markers is KRAS, which is a poor prognostic marker for survival and also indicates lack of response to EGFR TKIs. Histology is not a significant prognostic indicator, but it does affect treatment sensitivities and possible adverse effects. NSCLC mainly comprises adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Each of these is discussed in more detail in the pathology section, but for purposes of treatment, histology for NSCLC is generally divided into squamous and non-squamous histology since certain chemotherapies are less efficacious or contraindicated in the squamous subtype. Pemetrexed is a thymidylate synthase inhibitor often used as part of a platinum doublet and is considered a chemotherapy backbone in the treatment of NSCLC. However, it appears to be less effective in squamous cell histology. A large trial consisting of 1.700 treatment-naïve patients with advanced NSCLC compared cisplatin/pemetrexed to cisplatin/gemcitabine in the first-line setting. Investigators found that the OS was similar in both arms (median survival, 10.3 vs. 10.3 months, respectively; hazard ratio [HR] = 0.94; 95% CI 0.84-1.05), but that the pemetrexed arm had fewer toxicities. However, subset analysis showed OS was significant for the cisplatin/pemetrexed arm compared to the cisplatin/gemcitabine arm mainly in patients with adenocarcinoma (12.6 vs. 10.9 months, respectively) and large cell carcinoma histology (10.4 vs. 6.7 months, respectively). Meanwhile, those with squamous cell histology had significant improvement in survival with the cisplatin/gemcitabine arm over the cisplatin/pemetrexed arm (10.8 vs. 9.4 months, respectively).90 This difference in response may be due to higher expression of thymidylate synthase in the squamous subtype.91 Many other studies have confirmed this result, leading to the approval of pemetrexed for non-squamous NSCLC. Another agent not approved for treatment in squamous cell histology is bevacizumab, which is a recombinant monoclonal antibody that inhibits vascular endothelial growth factor (VEGF) and blocks angiogenesis. Its use was evaluated in the ECOG 4599 study, which assigned patients to either carboplatin/paclitaxel or carboplatin/paclitaxel and bevacizumab every 3 weeks. Median survival and median duration of PFS for the chemotherapy plus bevacizumab arm were superior to the chemotherapy-alone arm. However, this study was restricted to non-squamous cell carcinoma due to increased bleeding seen in the preceding phase 2 study.92 Since bleeding is a major complication of bevacizumab, the study patients also did not have brain metastases, hemoptysis, bleeding disorders, or anticoagulation requirements, though other studies have questioned some of these restrictions. The dangers of bevacizumab in squamous cell histology were thought to be due to the central location of these tumors and their proximities to vascular structures, but these do not appear to be definite, independent risk factors. In the Avastin in Lung (AVAiL) trial in which patients were assigned to cisplatin/gemcitabine with placebo, cisplatin/gemcitabine with 7.5 mg/kg of bevacizumab every 3 weeks, or cisplatin/gemcitabine with 15 mg/kg of bevacizumab every 3 weeks, central lesions were present in 38% of patients and did not appear to increase the rate of pulmonary hemorrhage.93 The trial also had 86 patients who developed venous thromboembolism requiring therapeutic anticoagulation and found that despite a higher bleeding rate in these patients, the risk appeared to be independent of whether the patient was receiving bevacizumab.94 Last, the PASSPORT trial used chemotherapy plus bevacizumab in 115 patients with brain metastases and had no cases of CNS hemorrhage.95 Still, the risk of bleeding is present. Despite the restrictions in ECOG 4599, patients receiving bevacizumab still had more bleeding compared to those who did not receive bevacizumab (4.4% vs. 0.9%; p = .001). Of the 15 treatment-related deaths in the bevacizumab group, 5 were from pulmonary hemorrhage. Thus, bevacizumab is contraindicated in those with squamous cell histology, and its serious side effects should be properly discussed with all other patients. Treatment in metastatic NSCLC is highly dependent on histology and whether the cancer has driver mutations, the PD-L1 status, and performance status. Specific TKIs are available for each driver mutation and are discussed separately in the TKI section. It is important to note, however, that when specific driver mutations such as EGFR, ALK, and ROS1 are present, treatment should be with the corresponding TKI regardless of PD-L1 status. With the rapid success and subsequent approval of many immunotherapies, chemotherapy has moved away from being the first-line treatment in metastatic NSCLC. Instead, immunotherapy is now preferred alone or in combination with chemotherapy, depending on the PD-L1 status of treatment-naïve patients without targetable mutations. Immune checkpoint inhibitor antibodies inhibit PD-1 (programmed cell death protein 1) receptors expressed on activated cytotoxic T cells or PD-L1 to improve immunity against tumors. For this reason, immunotherapy is contraindicated in those with autoimmune disorders and those who are on high doses of immunosuppressive agents. The immune checkpoint inhibitors currently available are pembrolizumab, nivolumab, durvalumab, and atezolizumab. KEYNOTE-024 was a landmark trial involving previously untreated advanced squamous or non-squamous NSCLC patients who had PD-L1 expression of at least 50% with no other targetable mutations. They were randomized to either pembrolizumab 200 mg every 3 weeks or the investigator’s choice of platinum-based chemotherapy. The respective median PFS (10.3 vs. 6.0 months) and OS (80.2% vs. 72.4% at 6 months) were both statistically significant in favor of pembrolizumab. The response rate was also higher (44.8% vs. 27.8%), the median duration of response (DOR) longer (not reached vs. 6.3 months), and the treatment-related adverse events (TRAEs) fewer (73.4% vs. 90.0%) compared to the chemotherapy arm. Thus, pembrolizumab was approved as first-line monotherapy in those with PD-L1 greater than or equal to 50%.96 Given these significant outcomes in patients with high PD-L1 expression, there was understandable curiosity regarding those who had less than 50% PD-L1. Another trial, KEYNOTE-042, followed again with treatment-naïve patients randomized to pembrolizumab versus platinum-based chemotherapy as first-line therapy but now included patients with PD-L1 of 1% or greater. These patients also had no actionable mutations. Again, there appeared to be greater benefit to the pembrolizumab arm, but subgroup analysis showed OS was mainly improved in those with PD-L1 levels of 50% or more, while those with PD-L1 tumor proportion score (TPS) of 1%-49% had similar OS to the chemotherapy group. However, this trial has since been updated with data in favor of pembrolizumab over chemotherapy. The patients were divided according to PD-L1 TPSs of 50% or greater, 20% or greater, and 1% or greater with the median OS for pembrolizumab compared to chemotherapy 20.0 versus 12.2 months, 17.7 versus 13.0 months, and 16.7 versus 12.1 months, respectively. The HR was 0.82 in favor of pembrolizumab in all 3 subgroups. The pembrolizumab groups also had better objective response rates and median DOR.97 The investigators concluded that single-agent pembrolizumab can be extended to include those without targetable mutations and with PD-L1 of 1% or greater in the first-line setting. However, this is still a category 2B according to the NCCN guidelines, with the only category 1 recommendation for single-agent pembrolizumab being in those with PD-L1 50% or greater. Using pembrolizumab alone for patients with PD-L1 greater than or equal to 50% is applicable to both squamous and non-squamous histology. For those with PD-L1 of 49% or less, however, the recommended treatments now actually consist of immunotherapy in combination with chemotherapy; regimen selection is based on histologic subtype. KEYNOTE-021 was a phase 2 trial that ultimately changed the landscape of treatment for advanced NSCLC. It randomly assigned untreated patients with stage IIIB/IV non-squamous NSCLC without an EGFR or ALK mutation to receive carboplatin/pemetrexed with or without pembrolizumab. The primary endpoint of overall response rate (ORR) showed superiority with the triple regimen compared to the doublet carboplatin/pemetrexed (55% vs. 26%; p = .0016).98 This trial was followed up with the phase 3 KEYNOTE-189 trial, which permanently entrenched this regimen into the algorithm for treating metastatic non-squamous NSCLC. The study involved 616 previously untreated patients with metastatic non-squamous cancer without sensitizing EGFR or ALK mutations. They were randomized to receive platinum and pemetrexed along with either pembrolizumab or placebo every 3 weeks for 4 cycles, followed by pemetrexed plus either pembrolizumab or placebo for up to 35 cycles. At the median follow-up of 10.5 months, the estimated rate of OS was an overwhelming 69.2% in the pembrolizumab arm compared to 49.4% in the placebo arm. OS improvements were seen in all subgroups regardless of PD-L1 status. The median PFS in the pembrolizumab group compared to the placebo was 8.8 months versus 4.9 months, respectively. The rate of grade 3 and above adverse events (AEs) was similar, if not slightly higher, in the triple-regimen group compared to the placebo (67.2% vs. 65.8%, respectively).99 Still, these findings resulted in approval by the Food and Drug Administration (FDA) of platinum/pemetrexed/pembrolizumab as first-line treatment in patients with non-squamous NSCLC without sensitizing EGFR or ALK mutations who otherwise have no contraindication to immunotherapy. With the success of this combination, many looked for other chemoimmunotherapy combinations that could be used in this patient population. IMpower150 was another phase 3 trial that looked at immunotherapy in addition to chemotherapy in patients with untreated metastatic non-squamous NSCLC. It differed from the KEYNOTE studies in that it used atezolizumab as the immunotherapeutic agent instead of pembrolizumab and also added bevacizumab. Patients were randomized to receive atezolizumab plus carboplatin/paclitaxel (ACP), bevacizumab plus carboplatin/paclitaxel (BCP), or atezolizumab plus bevacizumab with carboplatin/paclitaxel (ABCP). Treatments were given every 3 weeks for 4-6 cycles, followed by maintenance therapy with atezolizumab, bevacizumab, or both. Median PFS was longer in the four-drug regimen (ABCP) compared to the BCP group (8.3 vs. 6.8 months; HR 0.62, 95% CI 0.52-0.74, p < .001). PFS and median OS were also longer in this group compared to BCP regardless of PD-L1 status, thus making ABCP another viable option for metastatic non-squamous NSCLC (Figure 15-6).100 Figure 15-6. First-line treatment options for metastatic NSCLC without targetable mutations. Immunotherapy can still be used in combination with chemotherapy for metastatic squamous NSCLC, but special consideration has to be given when selecting chemotherapy agents due to the limitations of chemotherapy in this histology, as described previously. Investigators again added pembrolizumab to chemotherapy but could not use pemetrexed in this population. Instead, in KEYNOTE-407, these patients were randomized to receive carboplatin with either paclitaxel or nab-paclitaxel for the first 4 cycles in addition to either pembrolizumab or placebo for up to 35 cycles. The median OS was 15.9 months in the pembrolizumab arm compared to 11.3 months in the placebo arm. Again, this was seen regardless of the PD-L1 expression. Median PFS was also improved at 6.4 months in the pembrolizumab group versus 4.8 months in the placebo group.101 Similar to the trajectory of research regarding chemoimmunotherapy in non-squamous NSCLC, many also looked at the addition of atezolizumab to standard chemotherapy in squamous NSCLC. The IMpower131 study evaluated atezolizumab with carboplatin and paclitaxel or nab-paclitaxel in stage IV squamous NSCLC. Interim analysis showed improved PFS in the immunotherapy arm, and investigators presented an update at the European Society for Medical Oncology Conference in 2018, showing comparable OS. Data from this study are still maturing, and this regimen is currently awaiting FDA approval.102 We have discussed the benefits of adding immunotherapy, but what options exist if the patient has an active autoimmune disorder, for example, and is unable to be treated with immunotherapy? Chemotherapy alone still remains a reliable option. Data have shown that a platinum-containing regimen is superior to BSC in patients with incurable disease. Some studies have compared cisplatin to carboplatin in NSCLC and found cisplatin had a slightly better objective response rate and survival benefit (Table 15-2).103,104 TABLE 15-2 Trials Comparing Cisplatin Versus Carboplatin in Advanced NSCLC However, cisplatin is generally a tougher regimen, with common adverse effects including, but not limited to, neurotoxicity, usually in the form of peripheral neuropathy, nephrotoxicity, ototoxicity, and myelosuppression. Cisplatin also requires fluid administration before, during, and after treatment, with higher doses requiring more aggressive hydration. This poses a challenge in patients who are prone to fluid overload. Carboplatin is another platinum agent that is generally better tolerated than cisplatin. Its metabolism is 100-fold slower, as is its elimination. The dose of carboplatin is usually calculated to a target area under the curve (AUC) based on glomerular filtration rate (GFR). It can also cause peripheral neuropathy, renal toxicity, nausea/vomiting, and myelosuppression, but often to a lesser degree than cisplatin. For this reason, carboplatin is recommended for patients with multiple comorbidities or those who are unlikely to tolerate cisplatin. Combining a third-generation agent such as vinorelbine, paclitaxel, docetaxel, gemcitabine, and pemetrexed with a platinum agent improves survival rates compared to the same third-generation drug used alone (Table 15-3).9–11 Interestingly, this combination also showed better responses and improved survival compared to single-agent cisplatin alone (Table 15-4).12–14 TABLE 15-3 Trials Comparing Third-Generation Doublets Versus Single Agents TABLE 15-4 Trials Comparing Third-Generation Doublets Versus Single-Agent Cisplatin Thus, combining multiple drugs with different mechanisms of action and non-overlapping toxicities would improve the cytotoxic effects in theory. Numerous studies have demonstrated the feasibility of triplet combinations, with some showing good tolerability and activity, but most randomized trials have not shown a survival advantage with this regimen. Triplets also carry greater toxicity, so they are not routinely recommended for advanced NSCLC. Effective doublets in advanced NSCLC consist of a platinum agent in combination with paclitaxel or albumin-bound paclitaxel, docetaxel, gemcitabine, etoposide, or vinorelbine. Non-squamous NSCLC has the option of combining with pemetrexed. The ECOG 4599 trial mentioned previously found better medial survival with BCP compared to carboplatin/paclitaxel alone, but this improvement was only seen with the adenocarcinoma histology; overall, there were more significant toxicities in the bevacizumab arm.92 Another trial compared cisplatin/pemetrexed to cisplatin/gemcitabine in patients with stage IIIB or IV NSCLC. Those with adenocarcinoma or large cell carcinoma had improved survival with cisplatin/pemetrexed, while those with squamous cell carcinoma had improved survival with cisplatin/gemcitabine.105 Doublets not containing platinum may also be used, such as gemcitabine with docetaxel or vinorelbine. In patients with performance status 2 or greater, single-agent chemotherapy is also an option. These include single-agent paclitaxel, nab-paclitaxel, pemetrexed, docetaxel, or gemcitabine. Nab-paclitaxel is an albumin-bound form of paclitaxel whose active moiety is paclitaxel. It can be substituted for paclitaxel or docetaxel in patients who have hypersensitivity to either of these agents despite premedication or those who cannot receive premedications due to contraindications or other reasons. A trial comparing nab-paclitaxel/carboplatin to normal paclitaxel/carboplatin showed the former had less neurotoxicity and an improved response rate, leading to its FDA approval.106 Initial randomized studies did not show a survival difference with prolonged exposure to chemotherapy. One study randomized patients with advanced NSCLC to either 3 or 6 cycles of cisplatin, vinblastine, and mitomycin and found the median survival (6 months vs. 7 moths) and 1-year survival rates (22% vs. 25%, p = .2) were essentially the same.15 Not surprisingly, the patients randomized to a shorter course of chemotherapy also had less fatigue, nausea, and vomiting. Another study also had similar median survival and 1-year survival data using a carboplatin/vinorelbine doublet (Table 15-5).16 TABLE 15-5 Select Trials of Duration of Therapy for Advanced NSCLC However, more recent trials have challenged this and have shown a benefit with prolonged duration of therapy. Maintenance therapy refers to continued systemic therapy for advanced NSCLC after the initial 4-6 cycles of first-line chemotherapy. Patients may only receive maintenance therapy if their disease has remained stable or responded to treatment. There are 2 kinds of maintenance therapy: continuation and switch. Continuation maintenance refers to continuing with an agent that was already given in the first-line setting, for example, if a patient received cisplatin/pemetrexed initially for 4-6 cycles, then was continued on pemetrexed. This treatment is given until progression of disease or unacceptable toxicities. Both single-agent bevacizumab and single-agent pemetrexed are approved as maintenance therapies if used in the initial regimen.107–109 The combination of bevacizumab/pemetrexed is also an option but is currently a category 2A recommendation according to NCCN guidelines. Patients who received the 4-drug regimen of ABCP may be continued on atezolizumab, bevacizumab, or both.100 Similarly, patients who received platinum/pemetrexed/pembrolizumab may be continued on pembrolizumab/pemetrexed for maintenance (Table 15-6).99 TABLE 15-6 Selected Trials of Maintenance Therapy for Advanced NSCLC Switch maintenance refers to starting a different agent that was not included in the first-line regimen after completing the initial cycles. Trials had shown improved PFS and OS when pemetrexed was started as switch maintenance after 4-6 cycles of initial therapy in patients with non-squamous NSCLC, leading to FDA approval of maintenance pemetrexed.110 This is currently a category 2A recommendation for non-squamous histology, and docetaxel is a category 2B as switch maintenance in squamous cell carcinoma.111 Subsequent therapy for those with driver mutations is discussed in another section of this book. For all others, immune checkpoint inhibitors are preferred. These include nivolumab, pembrolizumab, or atezolizumab. If the patient still has progression of disease, however, switching to another immune checkpoint inhibitor is not routinely recommended. For patients who progressed on first-line therapy with pembrolizumab, subsequent treatment with a platinum-based doublet such as carboplatin/paclitaxel is recommended. For those who progressed on PD-1/PD-L1 inhibitors or chemotherapy, subsequent therapy is with chemotherapy. Docetaxel has shown improved survival and quality of life when compared to vinorelbine, ifosfamide, and BSC.112,113 It can be used alone or in combination with ramucirumab, which is a recombinant monoclonal antibody targeting VEGF. The REVEL trial compared ramucirumab/docetaxel to docetaxel alone in patients with metastatic NSCLC who had progressed. The combination showed a slight increase in medial OS (10.5 vs. 9.1 months; HR 0.86, 95% CI 0.75-0.98, p < .023) and was approved in the second-line setting for progression on or after platinum-based chemotherapy.114 Given its similarity to bevacizumab, ramucirumab also carries a risk of severe hemorrhage, gastrointestinal bleeding, gastrointestinal perforation or fistula, impaired wound healing, and poorly controlled hypertension. Last, gemcitabine and pemetrexed are also approved single-agent options for subsequent therapy (Figure 15-7). Figure 15-7. Subsequent treatment options in metastatic NSCLC without targetable mutations. Treatment of elderly patients is challenging due to their multiple comorbidities, but studies have shown they derive similar benefits from chemotherapy as younger patients. A phase 3 study randomly assigned patients older than 70 years to either BSC or weekly vinorelbine and found that those in the vinorelbine cohort scored better on quality-of-life scales and had fewer symptoms from their lung cancer.115 However, they also had more toxicity-related symptoms. Still, there was a significant median survival of 28 weeks for the chemotherapy group vs. 21 weeks for the other. An international panel for treatment guidelines in elderly patients with NSCLC had recommended single-agent chemotherapy with a third-generation drug, but more recently, studies have shown that a platinum-based doublet was still superior to single-agent regimens in fit elderly patients despite the increased toxicities.116 The Francophone de Cancerologie Thoracique conducted a study with patients ages 70 to 89 with ECOG performance status of 0 to 2 who were randomly assigned to either carboplatin/paclitaxel or single-agent vinorelbine or gemcitabine.117 Median OS was 10.3 months in the doublet arm compared to 6.2 months in the monotherapy arm (HR 0.64, 95% CI 0.62-0.78, p < .0001). Several subset analyses of other randomized trials found that older patients who received a platinum-based regimen had more treatment-related toxicities but overall had similar response rates and survival as compared to those for younger patients. Thus, age alone should not preclude patients from receiving appropriate treatment regimens. Patients with poor performance status (ECOG 2, 3, or 4) generally are less tolerant of treatment and have significantly shorter survival rates. Nevertheless, subset analyses suggest that patients with a performance status of 2 may still have a modest benefit from treatment, with studies favoring a doublet over a single agent if possible. The Cancer and Leukemia Group B (CALGB) 9730 trial randomized patients to single-agent paclitaxel versus carboplatin/paclitaxel.118 Patients with performance status (PS) of 2 did significantly worse than those with PS of 0 or 1, but of the patients with PS of 2, those treated with the doublet had a better survival rate than those treated with paclitaxel alone (median survival 4.7 months vs .2.4 months; 1-year survival = 18% vs. 10%; p = .0016). Another trial involving patients with Zubrod performance status of 2 compared carboplatin (AUC 5)/pemetrexed to pemetrexed alone and found improved median OS with the doublet compared to monotherapy (9.3 months vs. 5.3 months; p = .001).119 Thus, patients with ECOG 2 may still derive benefit from a doublet regimen, though treatment will depend on clinical judgment. One year after receiving treatment for a stage III adenocarcinoma of the lung, a 70-year-old male is found to have liver nodules. Core biopsy of one of the nodules was positive for adenocarcinoma of the lung primary. Next-generation sequencing revealed no actionable biomarkers. Immunohistochemistry (IHC) of the patient’s tumor expressed a PD-L1 TPS of greater than 50%. Learning Objectives: 1. Describe the role of PD-L1 TPS and tumor mutation burden in the treatment selection for patients with advanced NSCLC. 2. Compare the tolerability of immunotherapy versus systemic chemotherapy for patients with metastatic NSCLC. 3. Study the current first-line immunotherapy treatment options for patients with metastatic NSCLC Monoclonal antibodies against PD-1 and PD-L1 have changed the treatment paradigm in NSCLC since their first approval in 2015 by the USFDA, when nivolumab received granted approval for patients with metastatic NSCLC after progression on or after platinum-based chemotherapy.120 PD-1 is an inducible protein through T-cell receptors and cytokine receptors; it is expressed on the surface of all activated T cells, B cells, and natural killer cells. PD-1 regulates lymphocyte activation in lymphoid tissue, thereby controlling the magnitude of T-cell response during initiation and reactivation of the immune response, functioning as checkpoints to protect against self-reactivity.121 PD-L1 is expressed on antigen-presenting cells (APCs), induced by pro-inflammatory cytokines, including interferon 1 and 2, tumor necrosis factor α, and VEGF. Another ligand, PD-L2, is expressed on dendritic cells and macrophages induced by previous cytokines.122 Checkpoint inhibitors such as anti–PD-1 and PD-L1 antibodies have shown OS in both common lung cancer histologies adenocarcinoma and squamous cell carcinoma. This section of the chapter is dedicated to discussing and analyzing the evolution of these novel agents in the metastatic setting and how much we have to learn from monotherapy in second-line to single-agent or combo chemoimmunotherapy in first-line treatment for NSCLC. Due to improved OS over conventional cytotoxic chemotherapy as well as their relatively low-toxicity profile other than immune-related adverse events (ir-AEs), currently the immune-oncology approach has displaced chemotherapy to a place further down in the treatment algorithm of NSCLC. To make it easier for the reader, we start our review based on approval granted by the US FDA for each individual compound, and we discuss the initial clinical trials that granted approval by regulatory entities followed by the latest clinical trial in first-line therapy for patients with metastatic NSCLC. Nivolumab was the first drug to be approved by the US FDA for the treatment of metastatic NSCLC with progression of disease after platinum-based chemotherapy. Nivolumab is an immunoglobulin (Ig) G4 monoclonal antibody that has high affinity for the PD-1 receptor, blocking its interaction between ligands (PDL-1 and PDL-2), preventing T-cell inhibition. Nivolumab has been involved in multiple trials as first-line therapy in patients with metastatic NSCLC and as second- or third-line treatments following platinum-based chemotherapy (Table 15-7). TABLE 15-7 Randomized Clinical Trials Comparing Nivolumab in Patients With Stage IV NSCLC as Either First-Line Treatment or Second or Third Lines The CheckMate 057 phase 3 randomized controlled trial (RCT) involved patients with advanced NSCLC who progressed during or after platinum-based chemotherapy.123 Patients were randomized to receive either nivolumab or docetaxel, a chemotherapy still used as a “control arm” in second-line treatment for NSCLC. Patients with documented stage IIIB or IV and recurrent NSCLC following chemotherapy or radiotherapy and good ECOG performance status (0-1) were included in this trial. A total of 582 patients underwent randomization; 292 received nivolumab (3 mg/kg) and 290 docetaxel (75 mg/m2). Median age was 62 years, and minimum follow-up for OS was 13.2 months. Seventy-nine percent of patients reported current or former smoking status. PD-L1 protein expression was evaluated retrospectively and was further subclassified regarding specific expression levels in 1%, 5%, and 10% or higher. This study met its primary endpoint: OS. The OS was significantly longer during interim analysis, reporting a median OS of 12.2 months (95% CI 9.7-15) in the nivolumab group, in contrast to 9.4 months (95% CI 8.1-10.7) with docetaxel. The OS rate at 12 months was 51% (95% CI 45-56) with nivolumab versus 39% (95% CI 33-45) in the docetaxel group, and at 18 months, it was 39% (95% CI 34-45) with nivolumab and 23% (95% CI 19-28) with docetaxel. In a subgroup analysis, nivolumab was not favored in third-line therapy, CNS metastases, never-smoked status, and EGFR-positive status. The ORR was higher with nivolumab in comparison with docetaxel, 19% versus 12%, p = .002. PFS was 2.3 months (95% CI 2.2-3.3) with nivolumab and 4.2 months (95% CI 3.5-4.9) in the docetaxel group. Regarding PD-L1 expression, 78% of the patients (n = 455) had PD-L1 expression; nivolumab was associated with longer PFS, OS, and higher ORR than docetaxel, with PDL-1 expression levels of 1%, 5%, and 10% or higher.123 In terms of TRAEs, including grades 3 and 4, they were less prevalent in the nivolumab group than in the docetaxel group (7% to 20% any grade adverse effect, 5% to 18% grade 3 to 4) with median time of onset from 0.9 to 31.1 weeks in the nivolumab group. Eleven percent to 70% of ir-AEs were treated with glucocorticoids. Pneumonitis was the most common treatment-related serious adverse events (TRSAEs), which led to discontinuation of therapy in the nivolumab group. The CheckMate 017 phase 3, open-label trial compared nivolumab monotherapy with docetaxel single-agent therapy in patients with advanced squamous cell NSCLC after progression with platinum chemotherapy.5 Inclusion criteria were patients with stage IIIB or IV squamous cell NSCLC histology, disease recurrence after 1 prior platinum-containing regimen, and stable brain metastatic disease. A total of 227 patients underwent randomization; 135 patients were randomly assigned to receive nivolumab (3 mg/kg) every 2 weeks, and 137 patients were to receive docetaxel (75 mg/m2) every 3 weeks. PD-L1 expression was evaluated retrospectively and categorized in levels of 1%, 5%, or 10% of cells. The median age of the patients was 63 years; most patients had ECOG performance status score 1, stage IV, and were current or former smokers. Median follow-up was 11 months. The median OS was 9.2 months (95% CI 7.3-13.3) in the nivolumab group, in contrast to 6.0 months (95% CI 5.1-7.3) in the docetaxel group. OS was significantly better in favor of nivolumab, with a risk of death of 41% (HR 0.59, p < .001). The OS rate at 1 year was 42% (95% CI 34-50) in the nivolumab group versus 24% (95% CI 17-31) in the docetaxel group. HRs for death favored nivolumab across all subgroups except in the rest of the world region (Argentina, Australia, Chile, Mexico, and Peru) and in those 75 years or older. The ORR was significantly higher with nivolumab versus docetaxel (20% vs., 9%, p = .008). The PFS was 3.5 months (95% CI 2.1-4.9) in the nivolumab group and 2.8 months (95% CI 2.1-3.5) in the docetaxel group. The HR for death or disease progression was 0.62 (p < .001). The rate of PFS at 1 year in the nivolumab group was 21% versus 6% in the docetaxel group. Regarding PD-L1 expression, it was quantifiable in 83% of the patients and across the prespecified expression levels (1%, 5%, and 10%). PD-L1 was neither prognostic nor predictive of OS and PFS. The TRAEs were less frequent with nivolumab than with docetaxel. Grades 3 and 4 were less prevalent in the nivolumab group than for docetaxel (58% vs. 86% any grade AE, 7% to 55% grade 3 to 4). The most frequently reported TRAEs of any grade were hypothyroidism (4% nivolumab vs. 0% docetaxel), diarrhea (8% vs. 20%), pneumonitis (5% vs. 0%), and rash (4% to 6%). The TRAEs that led to discontinuation of treatment were less frequent in the nivolumab group than in the docetaxel group (3% vs. 10%, respectively); the most common AE per group was pneumonitis in the nivolumab group and fatigue in the docetaxel group.124 Recently, investigators of CheckMate 057 and 017 reported a 3-year follow-up on these studies. After a 40.3-month follow-up, nivolumab continued to show an OS benefit versus docetaxel: estimated 3-year OS rates were 17% (95% CI 14%-21%) versus 8% (95% CI 6%-11%) in the pooled population with non-squamous or squamous NSCLC. It is noteworthy that there were no new safety concerns identified. A total of 193 patients of 854 randomized patients across both studies had baseline liver metastases; nivolumab improved OS compared with docetaxel in patients with liver metastases (HR 0.68, 95% CI 0.50-0.91). This finding is consistent with the OS reported from the overall pooled study population (HR 0.70, 95% CI 0.61-0.81).125 In the CheckMate 026 clinical trial, 423 patients with untreated stage IV or recurrent NSCLC with PD-L1 tumor expression greater than 1% were randomized to receive either nivolumab- or platinum-based chemotherapy. Nivolumab was not associated with significantly longer PFS than chemotherapy among patients with a PD-L1 expression level of 5% or more. OS was similar between groups.126 The CheckMate 227 study is very important and innovative as it brought to the table a novel and potential biomarker: TMB.127 The CheckMate 227 trial has shown that the combination of nivolumab plus ipilimumab, a cytotoxic T-lymphocyte–associated protein 4 (CTLA4) inhibitor, significantly prolonged PFS versus chemotherapy among untreated patients with stage IV or recurrent NSCLC with high TMB regardless of PD-L1 expression. The analysis included 139 patients treated with nivolumab/ipilimumab (nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks) and 160 patients who received chemotherapy; randomization in 2 groups of TMB, < 10 and ≥ 10 mutations/megabase. As mentioned, these patients were treatment-naïve and negative for sensitizing EGFR or ALK mutations. Chemotherapy consisted of pemetrexed plus cisplatin or carboplatin, with optional pemetrexed maintenance in patients with non-squamous histology and gemcitabine plus cisplatin or carboplatin in patients with squamous histology. Those patients with a level of PD-L1 expression of at least 1% or above were randomly assigned in a 1:1:1 ratio to receive nivolumab plus ipilimumab, nivolumab monotherapy, or chemotherapy; those with a tumor PD-L1 expression level of less than 1% were randomly assigned in a 1:1:1 ratio to receive nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy. Co-primary endpoints were PFS and OS. Minimum follow-up was 11.2 months. Median PFS was 7.2 months in the nivolumab/ipilimumab group versus 5.5 months in the chemotherapy group (HR 0.58, p < .001), with 12-month rates of 42.6% versus 13.2%, respectively. Among 213 patients with PD-L1 expression of 1% or greater, the HR was 0.62 (95% CI 0.44-0.88). Among 86 patients with PD-L1 expression less than 1%, the HR was 0.48 (95% CI 0.27-0.85). Among 101 patients with squamous histology, the HR was 0.63 (95% CI 0.39-1.04). For those patients with non-squamous histology (n = 199), the HR was 0.55 (95% CI 0.38-0.80). The ORR was 45.3% in the nivolumab/ipilimumab group versus 26.9% for the chemotherapy group. Median DOR was not reached versus 5.4 months, with an ongoing response of more than 1 year observed in 68% of the nivolumab/ipilimumab group versus 25% in the chemotherapy patients. Among 380 patients with low TMB (<10 mutations/megabase) receiving either treatment in the trial, median PFS was 3.2 months in the nivolumab/ipilimumab patients versus 5.5 months in the chemotherapy group (HR 1.07, 95% CI 0.84-1.35).127 In terms of the safety profile, grade 3 or 4 TRAEs occurred in 31.2% of the nivolumab/ipilimumab group (n = 576 patients) versus 36.1% of the chemotherapy group (n = 570 patients), and TRSAEs occurred in 24.0% versus 13.9%, respectively. The most common ir-AEs in the nivolumab/ipilimumab group were skin reactions (32.4%), with the most common grade 3 or 4 events being hepatic events (8.0%). TRAEs led to discontinuation of treatment in 17.4% versus 8.9% of the patients, respectively. AEs led to death in 7 patients (1.2%) in the nivolumab/ipilimumab group (due to pneumonitis in 3 and 1 each with myocarditis, acute tubular necrosis, circulatory collapse, and cardiac tamponade) and in 6 patients (1.1%) in the chemotherapy group (due to sepsis in 2 and 1 each with multiple brain infarctions, interstitial lung disease, thrombocytopenia, and febrile neutropenia with sepsis).127 The other co-primary endpoint of this trial, OS, is not mature and is eagerly awaited. The current data of CheckMate 227 demonstrated that the nivolumab plus ipilimumab combination is superior than combination chemotherapy in terms of PFS in patients with TMB of more than 10 mutations/megabase. The second checkpoint inhibitor approved by the US FDA for lung cancer was pembrolizumab on October 2015. The initial approval was for patients with advanced/metastatic NSCLC whose disease had progressed after other treatments and with tumors that expressed PD-L1 analyzed via its companion diagnostic test, IHC 22C3. Pembrolizumab is a highly selective IgG4 monoclonal antibody against PD-1 that binds to the PD-1 receptor and inhibits its interaction with ligands PD-L1 and PD-L2, resulting in tumor recognition by T cells. The KEYNOTE-001 trial evaluated the safety, antitumor activity, and side effects of pembrolizumab in patients with advanced NSCLC.128 Inclusion criteria included patients who had locally advanced or metastatic NSCLC and ECOG performance status of 0-1. The study also looked to evaluate efficacy in patients with previously treated NSCLC with high expression of PD-L1 levels. A total of 495 patients were assigned to receive pembrolizumab at a dose of 2 mg/kg or 10 mg/kg every 3 weeks or 10 mg/kg every 2 weeks in the training group (182 patients) or validation group (313 patients). PD-L1 positivity was defined as staining in at least 1% (TPS) of cells within tumor nests or a distinctive staining pattern. Tumor response was assessed every 9 weeks via imaging. PD-L1 expression in at least 50% of tumor cells was selected as a cutoff for the training group.128 The ORR was 19.4% (95 CI% 16-23.2), including a response rate of 18% (95% CI 14.4-22.2) in 394 previously treated patients and 24.8% (95% CI 16.7-34.3) in the 101 previously untreated patients. The response rate was similar regardless of dose, schedule, and histologic analysis. At the time of analysis, 84.4% patients had no disease progression, with a median DOR of 12.5 months in all patients.128 Median PFS was 3.7 months (95% CI 2.9-4.1), and median OS was 12 months (95% CI 9.3-14.7) for all patients. The response rate was 45.2% (95% CI 33.5-57.3) in 73 patients with a TPS of at least 50%, and among all patients with a TPS of at least 50%, median PFS was 6.3 months. The median OS was not reached (Table 15-7). Treatment-related adverse events occurred in 351 patients (70.9%) with no clear difference according to dose or schedule; the most common AEs included fatigue, pruritus, and decreased appetite. Grade 3 or higher AEs were reported in 9.5% of patients. Ir-AEs occurred in more than 2% of patients and presented as infusion-related reaction (3%), hypothyroidism (6.9%), and pneumonitis (3.6%). The KEYNOTE-010 trial was a multicenter, open-label, phase 2/3 trial that evaluated pembrolizumab at a dose of 2 mg/kg or 10 mg/kg every 3 weeks versus docetaxel 75 mg/m2 every 3 weeks for PD-L1-positive NSCLC that progressed after platinum-based chemotherapy.129 Patients enrolled in this trial were at least 18 years old with progression of disease after 2 or more cycles of platinum-doublet chemotherapy and appropriate TKI on patients with EGFR mutation or ALK gene rearrangement, ECOG performance status 0-1, and PD-L1 expression of at least 1% TPS. Patients with previous treatment with PD-L1 inhibitor, brain metastases or carcinomatous meningitis, autoimmune disease on glucocorticoid treatment, and interstitial lung disease history of pneumonitis were excluded. Radiographic imaging was performed every 9 weeks, and response was assessed as per response criteria in solid tumors (RECIST) version 1.1. Primary outcomes were OS and PFS in the intent-to-treat (ITT) population and in patients with TPS of 50% or more. A total of 1,034 patients were enrolled; 345 received pembrolizumab at 2 mg/kg, 346 received pembrolizumab at 10 mg/kg, and 343 were allocated to a docetaxel arm. Most patients were current or former smokers, had non-squamous histology, and received one line of previous systemic treatment; few patients had EGFR-mutant or ALK-translocated tumors. PD-L1 TPS was referred as 1%-49% or more than 50% and evenly divided in the previous patient groups. Median follow-up was 13.1 months at the time of cutoff. At cutoff, median OS was 10.4 months with pembrolizumab at 2 mg/kg, 12.7 months with pembrolizumab at 10 mg/kg, and 8.5 months with docetaxel. The HR for pembrolizumab at 2 mg/kg versus docetaxel was 0.71 (95% CI 0.58-0.88, p = .0008), and the HR comparing pembrolizumab at 10 mg/kg versus docetaxel was 0.61 (95% CI 0.49-0.75, p < .0001). OS was similar in the 2 pembrolizumab groups in patients with PD-L1 TPS of 50% or higher (2 mg/kg vs. 10 mg/kg; HR 1.12, 95% CI 0.77-1.62). The PFS was longer with pembrolizumab at 2 mg/kg compared to docetaxel in patients with TPS of 50% or greater with a HR 0.59 (95% CI 0.44-0.78, p = .0001) and pembrolizumab at 10 mg/kg (95% CI 0.45-0.78, p < .0001) versus docetaxel. For the entire population, PFS was not statistically significant and did not differ by tumor histology (Table 15-7).129 Among patients with TPS of 50% or greater, median OS was significantly longer with pembrolizumab at 2 mg/kg than with docetaxel (14.9 months vs. 8.2 months, p = .0002) and pembrolizumab at 10 mg/kg than with docetaxel (17.3 months vs. 8.2 months, p < .0001). Grade 3-5 AEs occurred in 43 (13%) patients in the pembrolizumab group at 2 mg/kg, in 55(16%) patients in the pembrolizumab group at 10 mg/kg, and in 109 (35%) patients assigned to the docetaxel group. In the setting of ir-AEs, the most common ones were hypothyroidism, hyperthyroidism, and pneumonitis.129 KEYNOTE-010 was the platform to launch the KEYNOTE-024 trial; it included only patients with TPS ≥ 50%; this open-label, randomized, phase 3 trial compared pembrolizumab at 200 mg (flat dose) every 3 weeks with the investigator’s choice of platinum-based chemotherapy as first-line treatment for patients with NSCLC with high-expression PD-L1, defined by a TPS score of 50% or greater.96 Patients enrolled in this trial were 18 years or older, had histological or cytological confirmation of stage IV NSCLC with no EGFR mutations or ALK translocations, were naïve to systemic therapy for metastatic disease, had an ECOG PS of 0-1, and had a life expectancy of at least 3 months. Patients receiving systemic glucocorticoids or immunosuppressive therapy, untreated brain metastases, interstitial lung disease, or history of pneumonitis were excluded. Patients were randomly assigned in a 1:1 ratio to receive either pembrolizumab at 200 mg every 3 weeks for 35 cycles or the investigator’s choice of 1 of 5 platinum-based chemotherapy regimens for 4-6 cycles (carboplatin plus pemetrexed, cisplatin plus pemetrexed, carboplatin plus gemcitabine, cisplatin plus gemcitabine, or carboplatin plus paclitaxel). Pemetrexed regimens were permitted only for patients who had non-squamous tumors. Those patients who did undergo chemotherapy, at disease progression could cross over to receive pembrolizumab. Imaging studies were obtained every 9 weeks, and response to treatment was assessed according to RECIST. The primary endpoint was PFS; OS, ORR, and safety were secondary endpoints. Median duration of follow-up was 11.2 months. In the chemotherapy group, 66 patients (43.7%) crossed over to pembrolizumab after disease progression. Median PFS was 10.3 months (95% CI 6.7-not reached) in the pembrolizumab group and 6.0 months (95% CI 4.2-6.2) in the chemotherapy group. PFS was significantly longer in the pembrolizumab group than in the chemotherapy group (HR 0.50, p < .001). Regarding OS, there was a 40% decrease in mortality in favor of the pembrolizumab group (HR 0.60, p = .0050).96 The ORR was 44.8% (95% CI 36.-53.0) in the pembrolizumab group and 27.8% (95% CI 20.8-35.7) in the chemotherapy group, with a median time of response of 2.2 months in both groups. Median DOR was not reached in the pembrolizumab group and was 6.3 months in the chemotherapy group. In terms of toxicity, TRAEs occurred in 73.4% of patients in the pembrolizumab group and 90.0% in the chemotherapy group; grade 3 to 5 AEs were reported as much as twice as often in the chemotherapy group versus the pembrolizumab group (53.3% vs. 26.6%, respectively). The most common grade 3, 4, or 5 AEs reported in the pembrolizumab group were diarrhea (3.9%) and pneumonitis (2.6%). ir-AEs occurred in 29.2% of the patients in the pembrolizumab group versus 4.7% in the chemotherapy group; grade 3 to 4 ir-AES were severe skin reactions (3.9%), pneumonitis (2.6%), and colitis (1.3%) (Table 15-8).96 TABLE 15-8 Randomized Controlled Trials Comparing Pembrolizumab in Patients With Stage IV
15
NON–SMALL CELL LUNG CANCER TREATMENT: METASTATIC
SURGERY FOR OLIGOMETASTATIC DISEASE NON–SMALL CELL LUNG CANCER
DEFINITION OF OLIGOMETASTATIC DISEASE
DIAGNOSIS OF OLIGOMETASTATIC DISEASE
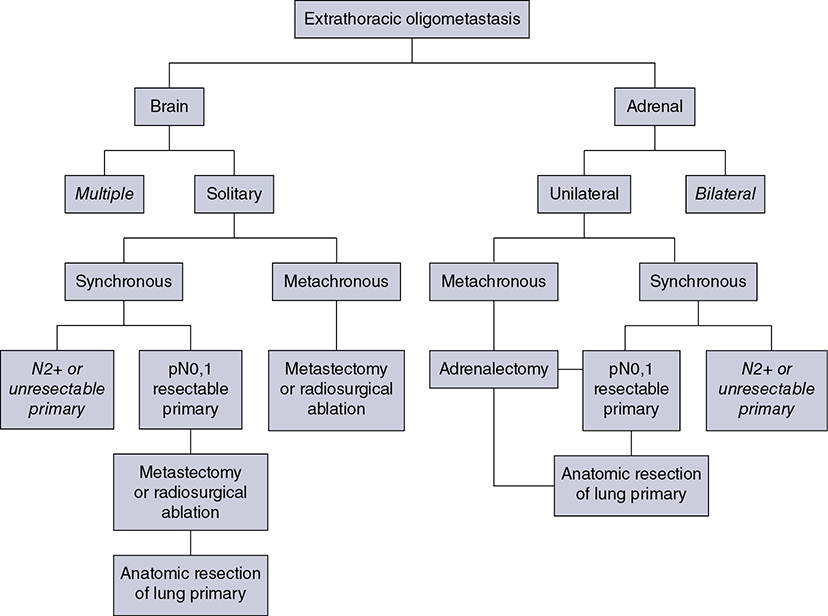
MULTIPLE PULMONARY LESIONS
MEDIASTINAL STAGING OF OLIGOMETASTATIC DISEASE
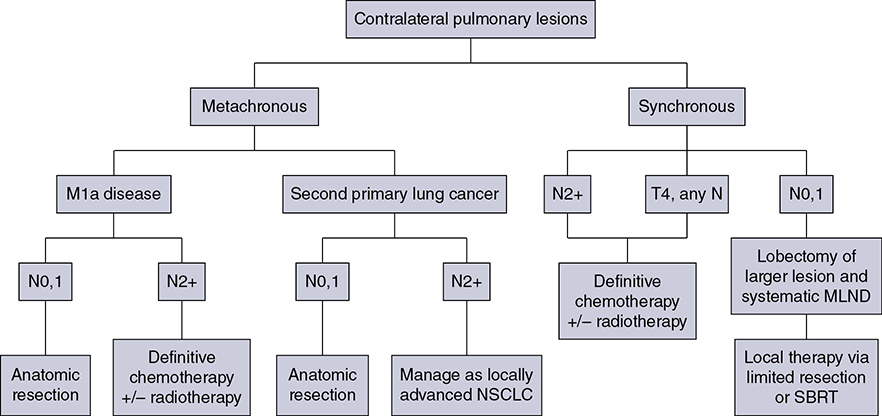
Low-Burden N2 Disease
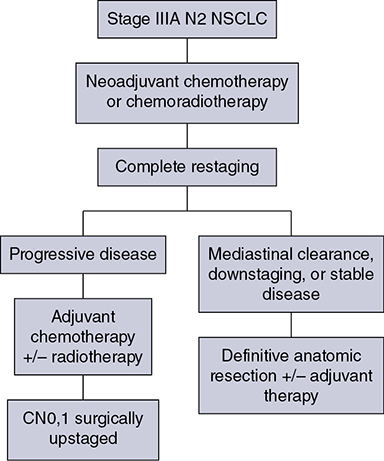
FUTURE PERSPECTIVE
RADIATION FOR METASTATIC NON–SMALL CELL LUNG CANCER
BONE METASTASES
PAIN CONTROL
FRACTIONATION
TOXICITY
RE-TREATMENT
VERTEBRAL BODY METASTASES
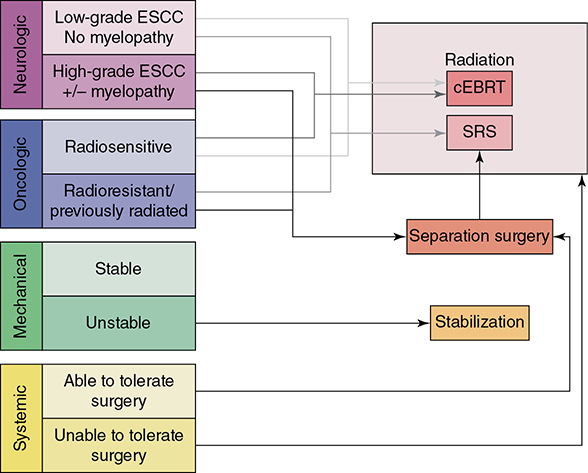
Neurologic Consideration
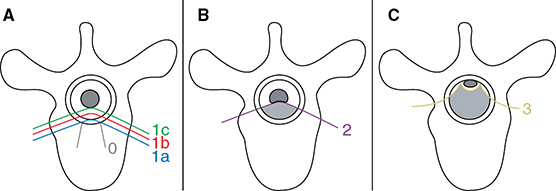
Oncologic Consideration
Mechanical Instability
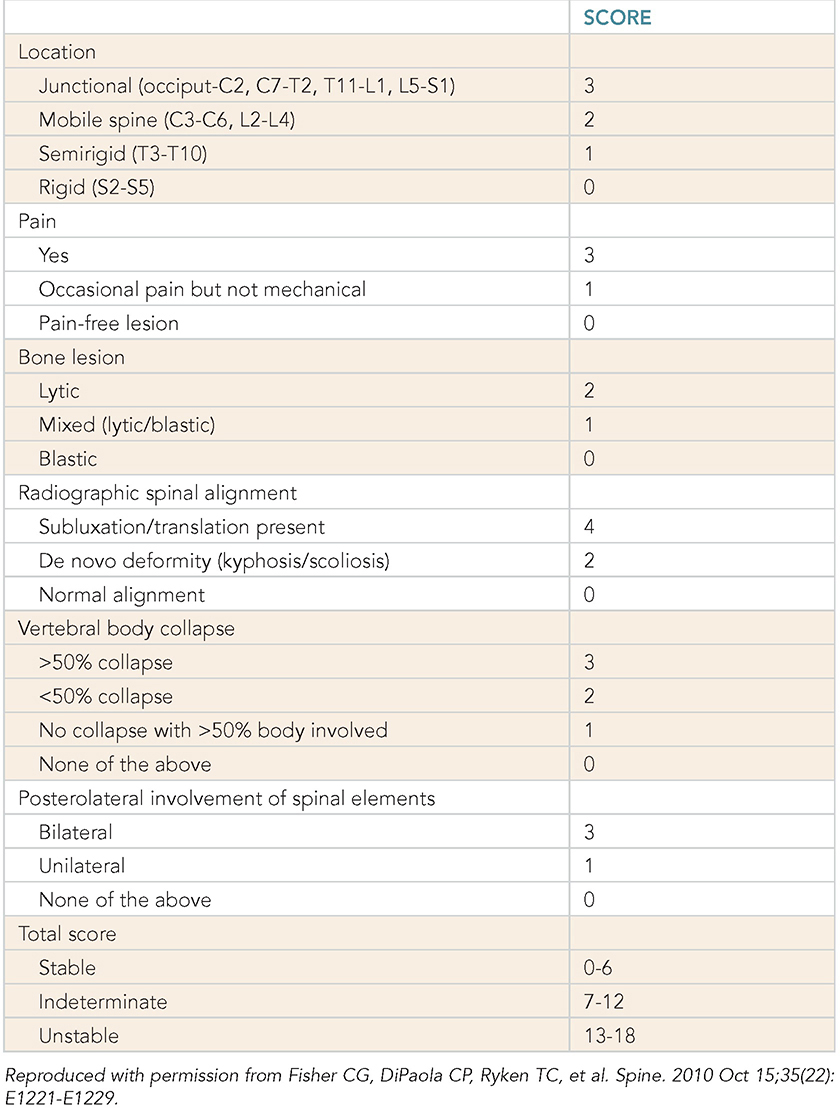
Systemic Assessment
SBRT VERSUS CONVENTIONAL RADIATION THERAPY
RADIONUCLIDES
OLIGOMETASTASES
WIDELY METASTATIC DISEASE
CNS METASTASES
CHEMOTHERAPY FOR METASTATIC NON–SMALL CELL LUNG CANCER
SELECTING TREATMENT REGIMENS
Driver Mutations
Histology: Squamous Versus Non-squamous
Pemetrexed
Bevacizumab
FIRST-LINE TREATMENT
Targeted Therapies
Chemotherapy Versus Immunotherapy
Single-Agent Immunotherapy
Combination Chemoimmunotherapy
NON-SQUAMOUS HISTOLOGY
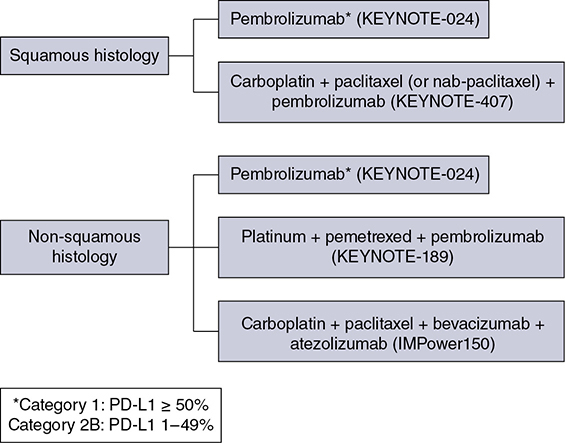
SQUAMOUS HISTOLOGY
CHEMOTHERAPY
Platinum Agents
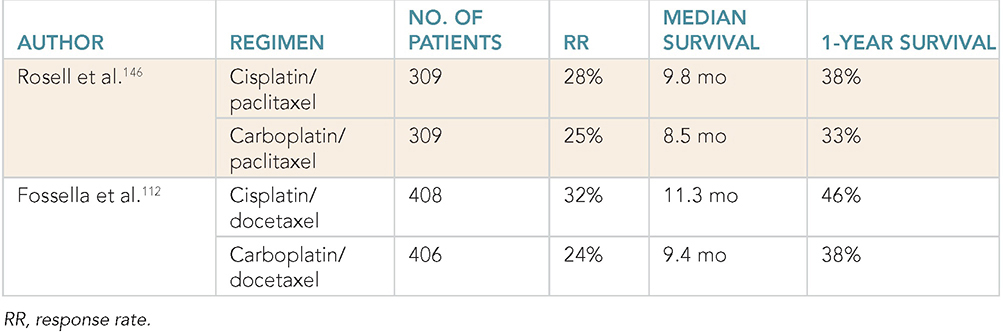
Standard Regimens
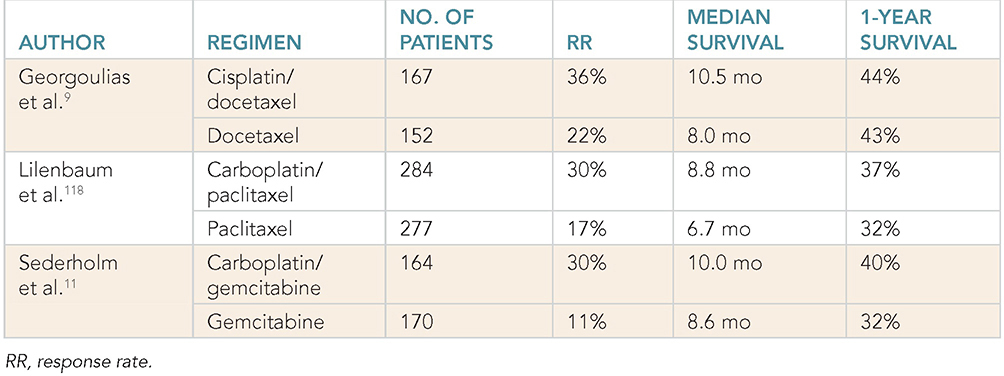
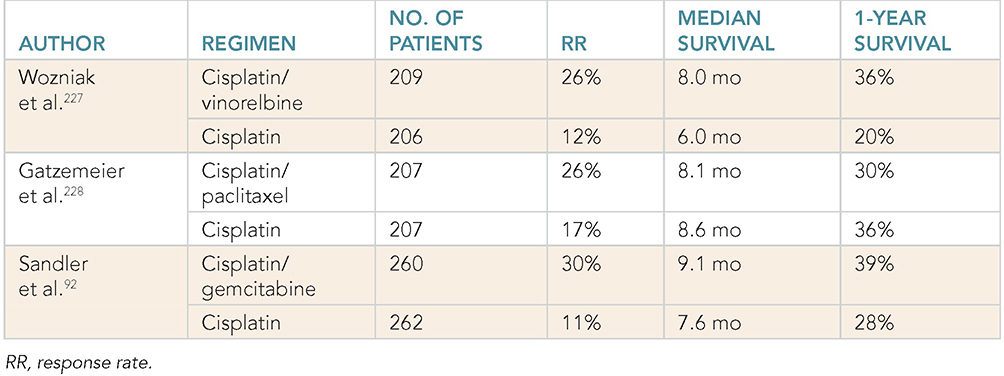
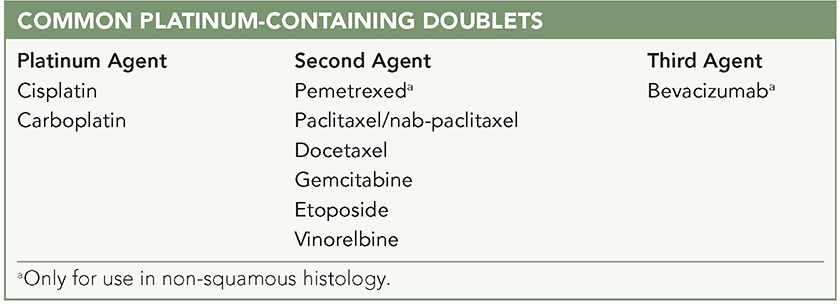
Nab-paclitaxel
MAINTENANCE THERAPY
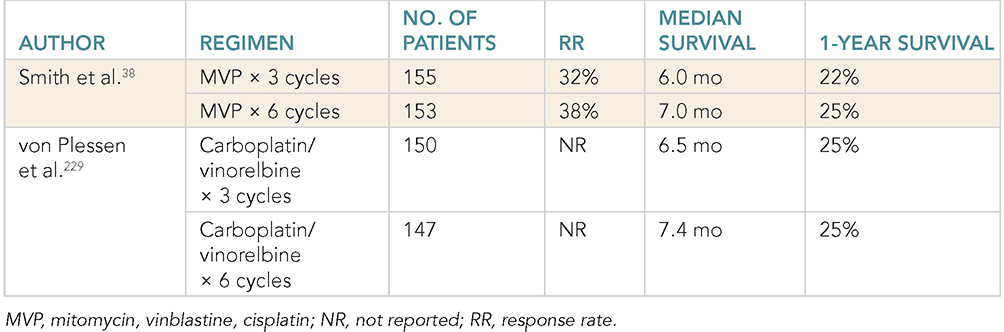
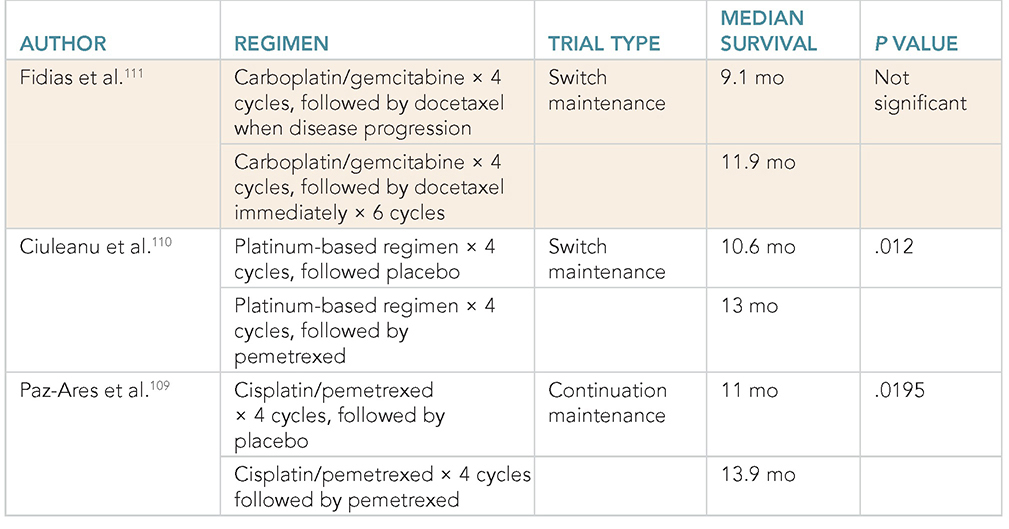
SUBSEQUENT THERAPY
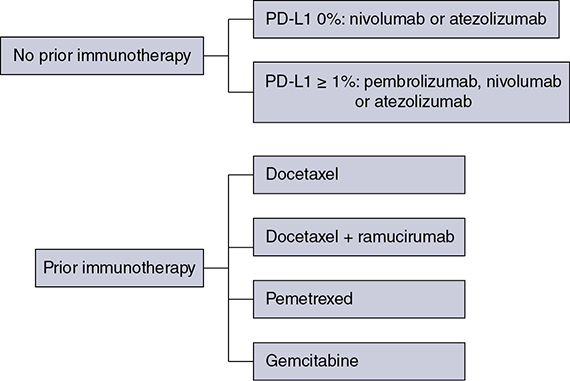
ELDERLY PATIENTS
POOR PERFORMANCE STATUS
IMMUNOTHERAPY FOR METASTATIC NON–SMALL CELL LUNG CANCER
NIVOLUMAB
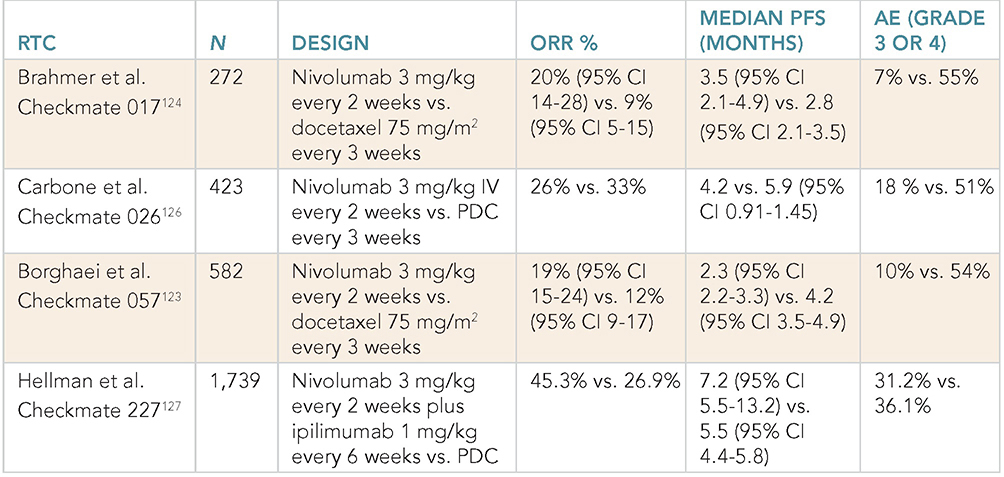
Second- or Third-Line Treatment: CheckMate 057 Trial
CheckMate 017 Trial
NIVOLUMAB AS FRONT-LINE THERAPY
CheckMate 026 Trial
CheckMate 227 Trial
PEMBROLIZUMAB
PEMBROLIZUMAB AS SECOND-LINE THERAPY OR BEYOND
KEYNOTE-001 Trial
KEYNOTE-010 Trial
PEMBROLIZUMAB AS FRONT-LINE THERAPY
Pembrolizumab as Monotherapy
KEYNOTE-024 Trial
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree